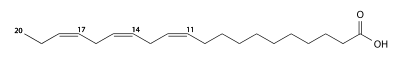
Eicosatrienoic acid
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Eicosatrienoic acid | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 20 H 34 O 2 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 306.50 g mol −1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Eicosatrienoic acid is a long-chain, polyunsaturated fatty acid in the omega-3 fatty acid group . The three double bonds are cis -configured, they are each separated by a methylene group . The trienoic acid is thus one of the isolenic acids .
It occurs esterified as triacylglyceride in small amounts in various types of conifers , but also in other plant species and in microalgae .
Isomers
There are several all- cis 20: 3 (ω − 6) isomers:
- Dihomopinolenic acid : 7,11,14-eicosatrienoic acid
- Dihomogammalinolenic acid : 8,11,14-eicosatrienoic acid
- Sciadonic acid : 5,11,14-eicosatrienoic acid
Further all- cis isomers are:
- Meadic acid : 5,8,11-eicosatrienoic acid, 20: 3 (ω − 9)
- 5,9,12-eicosatrienoic acid, 20: 3 (ω − 8)
- 5,9,13-eicosatrienoic acid, 20: 3 (ω − 7)
- 7,10,13-eicosatrienoic acid, 20: 3 (ω − 7)
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Francisco Pena-Pereira, Marek Tobiszewski: The Application of Green Solvents in Separation Processes. Elsevier, 2017, ISBN 978-0-12-805297-6 , p. 35.
- ^ John W. Blunt, Murray HG Munro: Dictionary of Marine Natural Products. Chapman & Hall, 2008, ISBN 978-08493-8216-1 , p. 742.
- ↑ Eicosatrienoic at PlantFA Database, accessed October 30, 2017.
- ↑ HM Rauen: Biochemical Pocket Book. 2nd edition, Springer, 1964, ISBN 978-3-642-85768-3 (reprint), p. 231.