Emerson reaction
The Emerson reaction [named after Edgar Emerson (née Eisenstaedt)] is a wet chemical method to identify characteristic structures and groups of certain molecules . It is widely used to determine the phenol content in wastewater (phenol index).
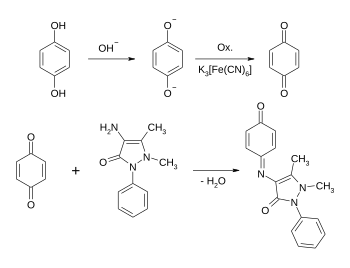
The reagent of the Emerson reaction is aminophenazone (4-aminoantipyrine), a pyrazolone skeleton known from fever and pain therapy with an amino group in the 4-position of the pyrazolone heterocycle. Often the reagent is just named aminophenazone. Due to the risk of confusion with the drug of the same name developed by Hoechst in 1896 , which has an N , N -dimethylamino group in the 4-position , this term should be avoided.
With the help of the Emerson reaction, activated aromatics , especially phenols, can be detected. The presence of an oxidizing agent such as potassium hexacyanoferrate (III) is necessary here.
The products of the Emerson reaction can also be quantitatively evaluated photometrically, in which case a conclusion can be drawn about the content of the sample.
The Emerson reaction is used, for example, in the analysis of acetylsalicylic acid . By-products from the synthesis ( acetylsalicylic anhydride ) can be detected here.
Individual evidence
- ^ Edgar Eisenstaedt: The Condensation of Aminoantipyrine with Aromatic Amines in the Presence of Oxidizing Agents . In: Journal of Organic Chemistry . tape 3 , no. 2 , 1938, p. 153-165 , doi : 10.1021 / jo01219a008 .
- ↑ Edgar Emerson: The Condensation of Aminoantipyrine. II. A New Color Test for Phenolic Compounds . In: Journal of Organic Chemistry . tape 8 , no. 5 , 1943, pp. 417-428 , doi : 10.1021 / jo01193a004 .
- ^ W. Pilz, Ilse Johann: III. Communication The reaction of phenol with 4-aminoantipyrine . In: Fresenius' Journal for Analytical Chemistry . tape 212 , no. 3 , January 1965, p. 410-419 , doi : 10.1007 / BF00519385 ( PDF ).
- ↑ DIN 38409-16 , June 1984.
- ↑ Entry on phenols. In: Römpp Online . Georg Thieme Verlag, accessed on December 29, 2014.