First substitute
| First substituents ( marked blue ) on benzene |
| Toluene : A blue- marked methyl group as the first substituent on the benzene ring. |
| Bromobenzene: A blue marked bromine atom as the first substituent on the benzene ring. |
| Nitrobenzene: A nitro group marked blue as the first substituent on the benzene ring. |
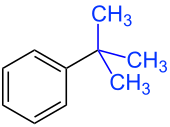
| tert -Butylbenzene: A blue- marked tert -butyl group as the first substituent on the benzene ring. |
A first substituent is the first and only substituent on an aromatic in organic chemistry . The aromatic is thus monosubstituted. If you subject z. B. a monosubstituted benzene derivative of an electrophilic substitution , the newly entering substituent can assume an ortho , meta or para position with respect to the first substituent. At the same time, the reaction rate of the electrophilic substitution of the monosubstituted benzene can be greater or less than that of benzene, depending on the nature of the first substituent.
If you electrophilically substitute toluene, a mixture of ortho- and para- substituted substitution products is formed. Starting from tert- butylbenzene, a mixture of ortho- and para- substituted substitution products is also formed . Since the first substituent in tert- butylbenzene is larger than the methyl group in toluene, the repulsive interactions between the electrophile and the first substituent result in less ortho and more para product.
Individual evidence
- ^ Peter Sykes: Reaction Mechanisms of Organic Chemistry , VCH, 9th revised edition, 1988, pp. 172-188, ISBN 3-527-26872-3 .
- ^ Ulrich Lüning: Organic reactions , 2nd edition, Elsevier GmbH, Munich, 2007, p. 89, ISBN 978-3-8274-1834-0 .