Flash vacuum pyrolysis
The flash vacuum pyrolysis ( FVP ), and flash vacuum pyrolysis is a chemical reaction in the high temperature and low pressure mostly gaseous substances decomposed be. A distinction is made between static and dynamic processes . In the former, the starting material is heated in a closed apparatus, such as in an autoclave or a sealed tube . In dynamic processes, a reaction mixture consisting of an inert gas and the starting material flows through either a heated reaction area or the starting material is evaporated in a high vacuum and thus introduced into the reaction area by molecular distillation.
Application in synthetic chemistry
Flash vacuum pyrolysis is mostly used as a process in synthetic chemistry. It was u. a. used by LT Scott to show the construction of geodetic domes and cages by rational synthesis. Scott FVP used as the last step of the synthesis of C 60 - fullerene . In this dynamic process of gas phase thermolysis , the substances evaporated in a vacuum are passed through a heated quartz glass thermolysis tube and then condensed in a receiver cooled with liquid nitrogen .
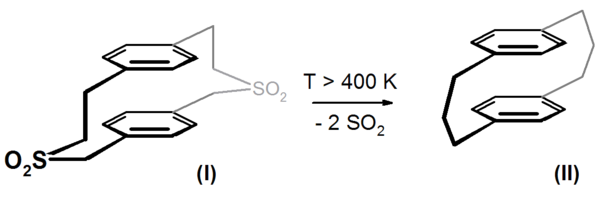
In a high vacuum, the reactant molecules are monomolecular in the pyrolysis zone; according to the dilution principle of Ruggli and Ziegler , intramolecular reactions predominantly occur. Oligo- and polymerization reactions are suppressed. An example is the synthesis of cyclophanes , u. a. successfully applied by Heinz A. Staab . Molecule (I) reacts under flash pyrolysis conditions with high yields with elimination of 2 sulfur dioxide molecules to form 3.3'-p, p'-cyclophane ( (II) ).
literature
- Anke Krüger: New carbon materials. An introduction , Vieweg + Teubner, 1st edition, Wiesbaden 2007, ISBN 978-3-519-00510-0 .
Individual evidence
- ↑ N. Hull: Thiete and Bisthiete in heterocyclic synthesis. (pdf) Dissertation at Johannes Gutenberg University Mainz , 2003.
- ^ GDCh : Organic Chemistry Trend Report 2002 ( Memento from February 23, 2005 in the Internet Archive ).
- ↑ B. Rosenau, C. Krieger, HA Staab: Tetrahedron Lett. , 26, 2081, 1985.