Gribble amination
The Gribble amination is a named reaction of organic chemistry , which was published by Gordon W. Gribble 1974th The amination is the synthesis of indolines or N -alkylated indolines. In contrast to other processes, this amination can produce indolines without polymerization .
Overview reaction
The synthesis of indolines or N -alkylated indolines takes place directly via an acidic reduction of indoles with sodium borohydride in acetic acid (H 3 C-COOH).
Alternatively, sodium cyanoborohydride and trifluoroacetic acid can also be used. In the reaction with trifluoroacetic acid, only indoline is formed without N -alkylation.
Reaction mechanism
In the proposed reaction mechanism, the amination for the synthesis of an N -alkylated indoline is described in three parts. The unalkylated indoline is already synthesized in the first part. In doing so, indole ( 1 ) is reduced to indoline ( 2 ):
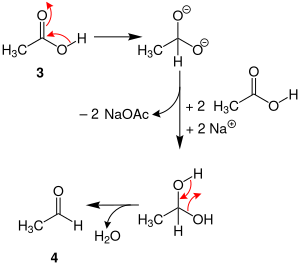
In the second part, acetic acid (AcOH) ( 3 ) is reduced to acetaldehyde ( 4 ) with elimination of water :
In the third part, indoline ( 2 ) reacts with acetaldehyde ( 4 ). The nitrogen lone pair of electrons attacks the carbonyl carbon atom of 4 . Intermediate product 5 is formed after dehydration . By attack of a hydride and subsequent protonation, the desired N -alkylated indoline 6 is formed:
modification
Gribble amination can also be used to reduce other heterocycles .
application
In general, this reaction is used to reduce indoles. However, it can also be used for the selective production of unsymmetrical tertiary amines .
Individual evidence
- ↑ a b c d e f Zerong Wang: Comprehensive Organic Name Reactions and Reagents . tape 1 . John Wiley & Sons, Inc., Hoboken, NJ, USA 2010, ISBN 978-0-471-70450-8 , pp. 1264-1266 , doi : 10.1002 / 9780470638859 .
- ↑ a b Gordon W. Gribble, Pierre D. Lord, Jerauld Skotnicki, Stephen E. Dietz, Jefferson T. Eaton: Reactions of sodium borohydride in acidic media. I. Reduction of indoles and alkylation of aromatic amines with carboxylic acids . In: Journal of the American Chemical Society . tape 96 , no. December 25 , 1974, p. 7812-7814 , doi : 10.1021 / ja00832a035 .
- ^ Brian Robinson: Reduction of indoles and related compounds . In: Chemical Reviews . tape 69 , no. 6 December 1969, p. 785-797 , doi : 10.1021 / cr60262a003 .