Grosheintz-Fischer-Reissert aldehyde synthesis
The Grosheintz-Fischer-Reissert aldehyde synthesis (also called Reissert aldehyde synthesis ) is used to produce aldehydes starting from the corresponding carboxylic acid chloride . The reaction is an extension of the Reissert reaction , in which an acid chloride is converted into the Reissert component. When the German chemist Arnold Reissert (1860–1945) first described the Reissert reaction in 1905, he also found that the Reissert component synthesized by him breaks down into the corresponding aldehyde in acid hydrolysis .
1941 noticed born in Germany chemist Hermann OL Fischer (1888-1960) and the Swiss-born chemist Jean M. Grosheintz that reissert reaction and the subsequent acid hydrolysis, as it was described by Reissert, just for aromatic components succeed . By changing the reaction conditions, they were able to synthesize not only aromatic but also aliphatic aldehydes from the corresponding acid chlorides. The Grosheintz-Fischer-Reissert aldehyde synthesis is therefore understood to mean a Reissert reaction with subsequent acidic hydrolysis, which can be carried out starting from aromatic and aliphatic acid chlorides and leads to the respective aldehydes.
Overview reaction
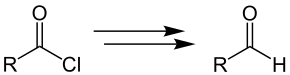
In the Grosheintz-Fischer-Reissert aldehyde synthesis, acid chlorides with an aromatic or aliphatic radical R are converted to the corresponding aldehydes:
The overview reaction can be divided into two parts in more detail. In the first step, the Reissert reaction, modified by Grosheintz and Fischer, of the acid chloride with an aliphatic or aromatic radical R to form the Reissert component and in the second step, the acid hydrolysis of the Reissert components to form the aldehyde.
The modified Reissert reaction marked in blue is discussed in more detail at this point: As described in the introductory text, it was only thanks to the changes made by Grosheintz and Fischer that not only aromatic acid chlorides but also aliphatic acid chlorides were able to produce the desired reaction. The key point of this modification was that the Reissert reaction must be carried out in benzene - a non-aqueous solvent . In addition, the stoichiometric ratios have been adjusted: One equivalent of the carboxylic acid chloride reacts with one equivalent of hydrogen cyanide and two equivalents of quinoline in absolute benzene:
Reaction mechanism
Many different reaction mechanisms have been proposed for the Grosheintz-Fischer-Reissert aldehyde synthesis. One possible mechanism is presented below.
The modified Reissert reaction takes place in the first part of the reaction mechanism. For these changes, however, no explicit information is available on the extent to which the mechanism of the Reissert reaction changes. However, it appears that the mechanism for the formation of the Reissert component will not change. The mechanistic details will not be discussed further at this point, see: Reissert reaction .
In the second part of the Grosheintz-Fischer-Reissert aldehyde synthesis, acid hydrolysis takes place. Initially, the mesomeric-stabilized Reissert components ( 2 ) form an intramolecular ring closure , resulting in ( 3 ). Then, as a result of protonation of the nitrogen atom, an imine ( 4 ) is formed, from which the zwitterion ( 5 ) is formed by deprotonation . After double protonation, the mesomeric stabilized component ( 6 ) is formed, from which the desired aldehyde ( 7 ) is obtained with the addition of water .
Atomic economy
The atom economy of the Grosheintz-Fischer-Reissert aldehyde synthesis can be classified as poor. In the context of this name reaction, large molecules (such as quinoline) are required, which occur in substituted form as an undesired by-product.
application
The Grosheintz-Fischer-Reissert aldehyde synthesis is used for the synthesis of aldehydes. The Rosenmund reaction is also used in this area . However, the aldehyde synthesis according to Grosheintz, Fischer and Reissert presented here should be more suitable than the Rosenmund reduction because the latter can lead to catalyst poisoning . An example of this is the synthesis of 3,4,5-trimethoxybenzaldehyde from 3,4,5-trimethoxybenzoyl chloride :
Individual evidence
- ↑ a b c d e f g h Z. Wang: Comprehensive Organic Name Reactions and Reagents. Volume 3, John Wiley & Sons, Hoboken 2009, ISBN 978-0-471-70450-8 , pp. 1284-1287.
- ↑ BP Mundy, MG Ellerd, FG Favaloro, Jr .: Name Reactions and Reagents in Organic Synthesis. 2nd Edition. Wiley-Interscience , Hoboken 2005, ISBN 0-471-22854-0 , p. 16.
- ↑ A. Reissert: About the introduction of the benzoyl group in tertiary cyclic bases. In: Reports of the German Chemical Society . Volume 38, No. 2, 1905, pp. 1603-1614, doi: 10.1002 / cber.19050380260 .
- ↑ A. Reissert: About the introduction of the benzoyl group in tertiary cyclic bases. (Second communication). In: Reports of the German Chemical Society . Volume 38, No. 3, 1905, pp. 3415-3435, doi: 10.1002 / cber.190503803170 .
- ↑ a b c J. M. Grosheintz, HOL Fischer: Preparation of 1-Acyl-1,2-dihydroquinaldonitriles and their Hydrolysis to Aldehydes. In: Journal of the American Chemical Society . Volume 63, No. 7, 1941, pp. 2021-2022, doi: 10.1021 / ja01852a066 .
- ^ A b R. L. Cobb, WE McEwen: Mechanism of the Acid-catalyzed Hydrolysis of Reissert Compounds. In: Journal of the American Chemical Society . Volume 77, No. 19, 1955, pp. 5042-5048, doi: 10.1021 / ja01624a031 .
- ↑ a b A. Schwartz: Efficient Synthesis of 3,4,5-Trimethoxybenzaldehyde via Reissert Aldehyde Synthesis. In: The Journal of Organic Chemistry . Volume 47, No. 11, 1982, pp. 2213-2214, doi: 10.1021 / jo00132a053 .