Rosenmund reduction
The Rosenmund reduction is a name reaction in organic chemistry that is named after the German chemist Karl Wilhelm Rosenmund (1884–1965). In this reaction, carboxylic acid chlorides react by catalytic hydrogenation to form corresponding aldehydes .
Overview
Carboxylic acid chlorides can be reduced to aldehydes with hydrogen in the presence of palladium . The palladium is previously selectively poisoned with quinoline and sulfur to prevent further reduction of the aldehyde.
mechanism
In the first reaction step, an organic palladium compound is formed. By hydrogenation are hydrogen chloride and palladium cleaved. The reaction product is an aldehyde.
If hydrogen is continuously passed through the reaction mixture, the hydrogen chloride formed can be driven off or bound by adding suitable bases (e.g. amines ). Palladium on the support barium sulfate serves as the catalyst.
Side reactions
Since a reduction down to alcohol can occur with untreated palladium , the activity of the catalyst is reduced by poisoning (e.g. with quinoline and sulfur). In some cases the acid chloride is reduced to the corresponding hydrocarbon.
Further by-products are the esters formed from the carboxylic acid chloride and the alcohols .
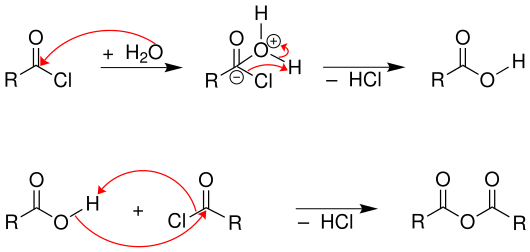
In addition, the reaction must be carried out with exclusion of water, since otherwise the acid chloride would be hydrolyzed to the corresponding carboxylic acid. Carboxylic acids react with acid chlorides to form carboxylic acid anhydrides .
literature
- Rosenmund, KW Reports of the German Chemical Society 1918 , 51, 585 doi : 10.1002 / cber.19180510170
- Saytzeff, Michael; Journal für Praktische Chemie 1873 , 6 (1), 128-135. doi : 10.1002 / prac.18730060111
swell
- Laue, T .; Plagens, A .: "Name and Keyword Reactions in Organic Chemistry". 3. Edition. Stuttgart: Teubner Study Books Chemistry (1998).
- HR Christen, Fundamentals of Organic Chemistry, Verlag Sauerländer Diesterweg Salle, p. 754.
Individual evidence
- ↑ T. Laue, A. Plagens: Name and catchword reactions of organic chemistry . Teubner Verlag, 2006, ISBN 978-3-8351-0091-6 , p. 290-292 .