Hofmann-Martius rearrangement
In chemistry, the Hofmann-Martius rearrangement is the acid-catalyzed rearrangement of N -alkylated aniline derivatives to give the corresponding ortho- and para -alkylated aniline derivatives. The reaction is mechanistically related to the Fries rearrangement . In practice, an inorganic acid , usually hydrochloric acid , is used in the heat .
The rearrangement reaction is named after its discoverers, the German chemists August Wilhelm von Hofmann (1818–1892) and Carl Alexander von Martius (1838–1920).
Overview reaction
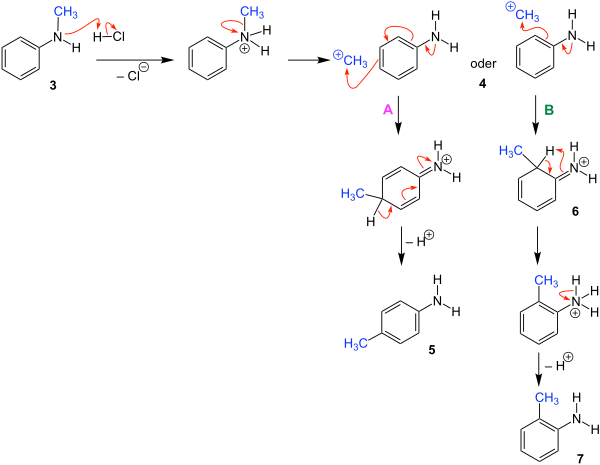
The reaction will be explained using the example of N -methylaniline . This leads to a rearrangement of the methyl group. The p -substituted aniline 1 and the o -substituted aniline 2 are obtained as products. An inorganic acid such as hydrochloric acid serves as the catalyst .
Instead of N -methylaniline, other N -alkylanilines can also be used, so the alkyl radical is variable.
Reaction mechanism
The following reaction mechanism is described in the literature and exemplified using N- methylaniline ( 3 ).
The proton of the hydrochloric acid first attaches itself to the amino function of the N -methylaniline ( 3 ), a hydrochloride is formed . Then a methyl radical splits off cationically from the amino group and the reactive intermediate 4 is formed as an intermediate . Now there can be two different electrophilic substitutions in 4 .
In route A, there is an addition of the methyl group in the para position. Then there is deprotonation and the product obtained is the para -substituted aniline 5 .
In route B, the methyl group is added to the ortho position and molecule 6 is formed. Through electron rearrangements in this molecule, hydrogen is added to the nitrogen. In the last step there is deprotonation and the ortho- substituted aniline 7 is obtained as the product .
variants
The Reilly-Hickinbottom rearrangement is known as a variant . Mechanistically, it proceeds in the same way, except that instead of inorganic acids, metal halides , mostly aluminum chloride , are used as Lewis acids .
Individual evidence
- ↑ AW Hofmann: Methylirung the phenyl group in the aniline . In: Reports of the German Chemical Society . tape 4 , no. 2 , June 1871, p. 742-748 , doi : 10.1002 / cber.18710040271 .
- ^ Zerong Wang: Comprehensive Organic Name Reactions and Reagents , Wiley, 2010, ISBN 9780470638859 , pp. 1469-1472, doi: 10.1002 / 9780470638859 .
- ↑ Joseph Reilly, Wilfred John Hickinbottom: Intramolecular rearrangement of the alkylarylamines: formation of 4-amino-n-butylbenzene . In: Journal of the Chemical Society, Transactions . tape 117 , no. 0 , 1920, p. 103-137 , doi : 10.1039 / CT9201700103 .