Conformity assessment
Conformity assessment is defined in the international standard ISO / IEC 17000 "Conformity assessment - terms and general principles" as "demonstration that specified requirements relating to a product, a process, a system, a person or a body are met".
General
Conformity assessment is an umbrella term for activities of selecting, determining (of properties), evaluating (e.g. for compliance with specified or general requirements) and confirming (e.g. by declaring the manufacturer or a certificate from a certification body that a product complies with certain standards). Such activities are, for example, sampling , testing, inspecting, explaining, certifying. The objects of conformity assessment are not restricted.
Conformity assessment takes place in a variety of ways and at all levels:
- in the company (e.g. final inspection, auditing of a quality management system by our own auditors ): >> First party
- by bodies / persons of the customer or recipient: >> Second Party
- by commercial conformity assessment bodies independent of the client (e.g. laboratories; certification bodies; inspection bodies; accreditation bodies ): >> Third Party
Conformity assessment takes place both on a legally unregulated basis (for example as a development-accompanying test or as confirmation of properties in a contractual relationship, so-called "voluntary area", such as certification as a " Bioland company ") and on the basis of legal regulations (so-called "regulated Area"). Conformity assessments in the regulated area can be voluntary (for example: the eco-audit) or a mandatory prerequisite for market access (for example: approval of medical devices according to the Medical Devices Act ).
EU conformity assessment
The conformity assessment is of particular importance. B. by notified bodies when evaluating products for their compliance with the requirements of an EU directive . Directive 2001/95 / EC on general product safety for the European internal market defines minimum safety requirements for numerous products that must be met by the manufacturer.
The manufacturer must use a “conformity assessment procedure” to prove that he has complied with the basic safety requirements contained in the directive or directives. The manufacturer must carry out the conformity assessment procedure for each product before it is placed on the market for the first time . At the end of the conformity assessment procedure, the manufacturer issues an EU declaration of conformity for his product, in which he declares that the product conforms to the requirements of the relevant directive (s). The manufacturer then attaches the CE mark to the product , if the applicable directive provides for this.
Only in the " Medical Devices " sector is there the special feature that not only product safety has to be proven within the scope of the conformity assessment, but also the medical-technical performance of medical devices, as advertised by the manufacturer in the product labeling including advertising as a medical indication . The corresponding verification procedure is called clinical evaluation . Only the evidence of product safety and performance, certified externally by notified bodies (depending on the product class), entitles manufacturers of medical devices to affix the CE mark.
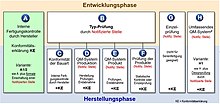
In the appendices to the guidelines, various modules for carrying out a conformity assessment procedure are named. Which modules can be chosen depends on the classification of the product. For products with a higher risk, the involvement of a Notified Body in the implementation of the conformity assessment procedure is mandatory.
Modules for proof of conformity
In each individual harmonization directive ( e.g. Pressure Equipment Directive 2014/68 / EU, Machinery Directive 2006/42 / EC, ATEX Directive 2014/34 / EU , Medical Products Directive 93/42 / EEC) the conformity assessment procedure to be used is described by specifying modules or module combinations in order to demonstrate conformity with the basic requirements applicable to the product. Possible modules or module combinations are ( not a complete list ):
- Module A
- Internal production control and proof of conformity by the manufacturer (this is the most common proof of conformity)
- Module B
- Variant 1: EC type examination (determination of the conformity of the type of product with the basic requirements of the directive (s) e.g. on the basis of the relevant harmonized standards or other relevant standards by the notified body )
- Variant 2: Design review (determination of the conformity of the technical documentation of the product with the basic requirements of the directive (s) by the notified body)
Module B is always to be used in combination with module C, D, E, or F:
- Module C
- Conformity with the design (the manufacturer declares the conformity of the series products with the tested type on the basis of its internal production control)
- Module D
- Quality assurance production (description of quality assurance measures before, during and after production including their frequency; audit by the notified body)
- Module E.
- Product quality assurance (description of the quality assurance measures after production including their frequency (final acceptance and testing); audit by the notified body)
- Module F
- Testing of the products (testing of the products for conformity with the type either through statistical controls or testing of each individual product by the notified body)
- Module G
- Individual test (by the notified body; only applicable to products that are not produced in series)
- Module H
- Comprehensive quality assurance (preferably applicable to products that are mass-produced; can be used as an alternative to module B in some guidelines; audit by the notified body)
- Module H1
- Comprehensive quality assurance (including checking the conformity of the draft and issuing an "EC design examination certificate"; audit and testing by the notified body)
With regard to the various procedures for the assessment (certification) of quality assurance systems , the manufacturer can choose between the following quality modules:
- Module D (quality assurance of production - manufacture, final acceptance and testing):
Assessment and evaluation by the notified body. Upon approval of the quality assurance system, issue of the EU Module D certificate. The quality assurance system always relates to specific equipment and requires a valid Module B certificate for the equipment in question. The manufacturer or his authorized representative is only entitled to affix the conformity mark in conjunction with both certificates .
- Module E (quality assurance of the product - final acceptance and testing):
The approval of the quality assurance system always relates to a specific piece of equipment and requires a valid Module B certificate for the piece of equipment in question. The manufacturer or his authorized representative is only entitled to affix the conformity mark in conjunction with both certificates.
The higher the hazard potential of a product, the greater the scope of testing that has to be transferred to a “ Notified Body ”. This is indicated by a four-digit code number after the CE mark . The manufacturer can carry out the conformity assessment for products with a very low risk potential himself without involving a notified body.
In any case - even if a third party is involved - an EU declaration of conformity (usually) must be issued by the manufacturer. This underlines his sole responsibility (= liability) for the product.
The safety requirements of the EU directives must be observed during the conformity assessment. These are usually quite general, so that specific technical requirements have to be derived from rules that reflect the generally recognized rules of technology . These can be German standards , European standards , standards of a member state of the EU or standards of associations. In the European single market , the proof of conformity of products and services with European legal requirements and with European standards is a regulatory and competitive instrument of considerable importance.
The European Commission has laid down its ideas on this in the “Global Concept for Certification and Testing in Europe”. It wants to give the conformity assessment uniform standards so that products no longer have to be subjected to multiple national tests and certifications in the interests of the free movement of goods . The conformity assessment is carried out in the scope of the guidelines according to the New Concept (or New Concept).
In order to achieve the goal of free movement of goods and trade and the standardization of test and administrative procedures, the Council decided in 1990 and 1993 on the modules to be used in the technical harmonization guidelines; since 2008 replaced by the "Decision 768/2008 / EC of the European Parliament and of the Council on a common legal framework for the marketing of products and repealing the Council Decision 93/465 / EEC" of July 9, 2008. This adjustment is part of the so-called " New Legislative Framework " (OJ L 218/82 of August 13, 2008).
literature
- BVMed information series "Medical product law": "Conformity assessment procedure for medical products"; www.bvmed.de.
- Günther Beer: Conformity Assessment - Terms and General Basics in DIN Communications, October 2005, Beuth Verlag
- DIN EN ISO 17000: Conformity assessment - terms and general principles
- Röhl, Hans Christian / Schreiber, Yvonne: Conformity Assessment in Germany , 2006
- Klaus-Peter Schulz: Keywords for European Standardization , Beuth, 2002, ISBN 3410152695