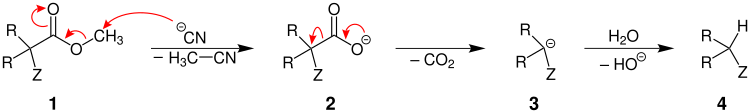Krapcho decarboxylation
The Krapcho decarboxylation , sometimes called the Krapcho reaction or Krapcho decarbalkoxylation , is a name reaction in organic chemistry . The reaction was named after its discoverer Andrew Paul Krapcho , who published it in 1967. With the aid of this reaction, α, α-disubstituted esters and α-monosubstituted esters can be decarboxylated .
Overview
The reaction takes place in an aprotic-polar solvent (e.g. DMSO , DMF , DMA or HMPT ). When esters, which have an electron-withdrawing radical in the α-position (shown here as X), react with cyanide ions (e.g. from KCN ), the ester is decarboxylated and carbon dioxide is released as a gas .
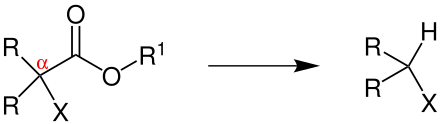 R = hydrogen , alkyl group , aryl group , the radicals can be the same but also different; R 1 = alkyl group; X = electron-withdrawing group, such as nitrile group , ester group , carbonyl group or sulfone group
R = hydrogen , alkyl group , aryl group , the radicals can be the same but also different; R 1 = alkyl group; X = electron-withdrawing group, such as nitrile group , ester group , carbonyl group or sulfone group
Instead of potassium cyanide, the reaction can also be carried out with other salts : NaCN , LiCl , NaCl , NaBr , NaI , LiI · H 2 O, Na 2 CO 3 · H 2 O, Na 3 PO 4 · 12 H 2 O.
mechanism
The course of the mechanism depends on the radicals R which are bonded to the α-carbon atom.
α, α-disubstituted esters
Are both radicals R is different from hydrogen, the ester is thus α, α-disubstituted, engages the salt (in this case by the cyanide anion shown), the alkyl group (here, a methyl group ) 1 in the course of a S N 2-reaction and goes as methyl cyanide from . What remains is a carboxylate 2 from which carbon dioxide is now split off by rearrangement of electrons and a carbanion 3 is formed as a transition state. This is protonated by water to give the decarboxylated product 4 .
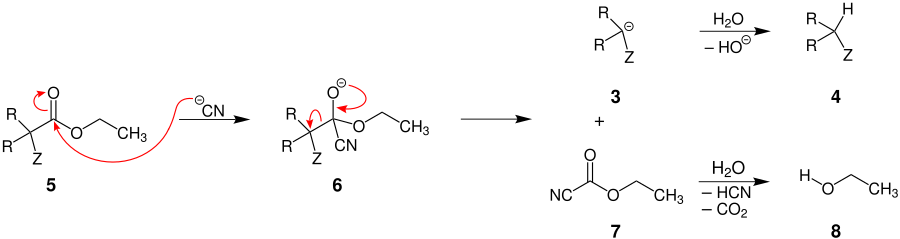
α-monosubstituted esters
If one of the radicals R = H, the starting material is an α-monosubstituted ester 5 . In this case the cyanide ion attacks the carbonyl group 5 and a tetrahedral transition state 6 is created . With the formation of a C = O double bond, the carbanion 3 is formed on the one hand , which is also formed in α, α-disubstituted esters, and on the other hand ethyl cyanoformate ( 7 ). The carbanion 3 is in turn protonated by water to form the product 4 . The ethyl cyanoformate ( 7 ) reacts with water with elimination of hydrogen cyanide and carbon dioxide to form the by-product 8 in the form of an alcohol.
Individual evidence
- ↑ a b c d Z. Wang: Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 1687 f.
- ↑ AP Krapcho, EGE Jahngen Jr., AJ Lovey: Decarbalkoxylations of geminal diesters and β-keto esters in wet dimethyl sulfoxide. Effect of added sodium chloride on the decarbalkoxylation rates of mono- and di-substituted Malonate esters . In: Tetrahedron Letters . tape 15 , no. 13 , 1974, p. 1091-1094 , doi : 10.1016 / S0040-4039 (01) 82414-X .
- ↑ AP Krapcho, JF Weimaster, JM Eldridge, EGE Jahngen Jr., AJ Lovey, WP Stephens: Synthetic applications and mechanism studies of the decarbalkoxylations of geminal diesters and related systems effected in dimethyl sulfoxide by water and / or by water with added salts . In: J. Org. Chem. Band 43 , no. 1 , 1978, p. 138-147 , doi : 10.1021 / jo00395a032 .
- ↑ AP Krapcho, GA Glynn, BJ Grenon: The decarbethoxylation of geminally dicarbethoxy compounds . In: Tetrahedron Letters . tape 8 , no. 3 , 1967, p. 215-217 , doi : 10.1016 / S0040-4039 (00) 90519-7 .
- ^ JJ Li: Name Reactions. A Collection of Detailed Reaction Mechanisms . 3rd expanded edition, Springer, Berlin / Heidelberg 2006, ISBN 978-3-540-30030-4 , p. 230.
- ↑ a b c d L. Kürti , B. Czakó: Stratigic Applications of Named Reactions in Organic Synthesis . Elsevier Academic Press, Amsterdam 2005, ISBN 978-0-12-429785-2 , p. 252.