Molar volume
| Physical size | |||||||
|---|---|---|---|---|---|---|---|
| Surname | Molar volume | ||||||
| Formula symbol | |||||||
|
|||||||
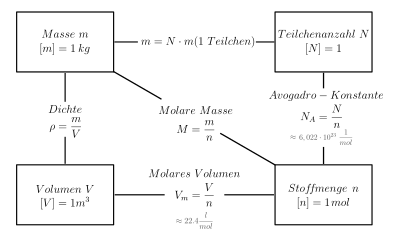
The molar volume or molar volume ( symbol : V m ) of a substance indicates the volume of a mole of this substance . Its SI unit is m 3 / mol.
It results from the quotient of the volume V and the amount of substance n and can be calculated from the molar mass M and the density ρ of the corresponding substance.
It describes the volume that is filled by a total of 6.022 · 10 23 particles ( Avogadro's constant ) of a substance . The molar volume is an intensive quantity , while the volume itself is an extensive quantity .
For solids and liquids , the molar volume depends on the respective substance ( substance property ). The molar volume, like the (mass) specific volume, is a standardized volume.
Molar volume of the ideal gas
By rearranging the ideal gas equation one obtains for the molar volume of an ideal gas :
- ,
With
- R is the universal gas constant
- T is the temperature (K) and
- p is the pressure (kPa) .
The molar volume of an ideal gas is, according to the current measurement accuracy ( regardless of which gas is present in detail ):
| conditions | pressure | temperature | Molar volume | annotation |
|---|---|---|---|---|
| Physical standard conditions | 101.325 kPa | 0 ° C | 22.413 969 54 ... 10 −3 m 3 / mol | " Standard volume " |
| Standard chemical conditions (IUPAC 1982) | 100,000 kPa | 0 ° C | 22.710 954 64 ... 10 −3 m 3 / mol | |
| SATP terms | 101,300 kPa | 25 ° C | 24.471 441 56 ... 10 −3 m 3 / mol. |
In the German standard DIN 1343, January 1990 edition, the molar standard volume of the ideal gas is still given with the older value of (22.414 962 ± 0.000 013) l / mol. The older value completely covers the younger one due to its greater uncertainty.
For real gases , the numbers from the ideal gas law only apply at low pressure and not too low temperatures.
See also
Individual evidence
- ↑ CODATA Recommended Values. National Institute of Standards and Technology, accessed July 30, 2019 . Value for the molar volume under standard conditions (273.15 Kelvin , 101.325 kPa ). The value is exact, but has an infinite number of decimal places in the decimal representation.
- ↑ CODATA Recommended Values. National Institute of Standards and Technology, accessed July 30, 2019 . Value for the molar volume at 273.15 Kelvin and 100 kPa . The value is exact, but has an infinite number of decimal places in the decimal representation.