Spherical tube
Ball tubes (also ball tubes) are devices made of glass, which - generally speaking - are used in the chemical laboratory, when it is necessary to bring into contact with gases or vapors in air exclusion solids or liquids; Vapors can also be condensed in spherical tubes. Glassblowers produce spherical tubes by blowing a glass tube that has been heated at certain points to soften it into a sphere. The ball can be at the end or in the middle of the glass tube. This can also be bent into various shapes, e.g. B. in U-shape.
History
In the 19th century in particular, spherical tubes were widely used in chemical laboratories. Therefore, the term "Kugelrohr" is still used today as a loan word in the English-speaking world.
If you put a substance in a bulb tube, it can be heated in the flow of a gas. Reactive gases, e.g. B. ammonia, chlorine, hydrogen chloride, oxygen, hydrogen can then react with the substance. Solids were heated and dried in a stream of an inert gas (e.g. argon, nitrogen); But there are better devices for that today. U-shaped spherical tubes can be cooled in cold baths so that liquid collects in the sphere.
Kugelrohr distillation
In the organic chemical laboratory, spherical tubes are used to separate small amounts of substances with a boiling point of less than 300 ° C by distillation . As a rule, the Kugelrohr distillation is carried out in vacuo in order to achieve a lower boiling point. Distillations in the Kugelrohr are appropriate if the amount of substance is too small to be managed with the devices for "simple distillation " (flask, distillation bridge ). As a rule, the Kugelrohr is used if one wants to distill approx. 1 to 3 ml of substance, but amounts of substance below 1 ml can also be distilled. The constitution and structure of the chemical compound can already be determined with approx. 50 to 100 mg of distillate (NMR spectrum, IR spectrum, mass spectrum).
A bulb tube distillation is particularly suitable for isolating substances from fractions of chromatographic separations and for removing highly volatile solvent residues. Many syntheses in the research laboratory are now carried out on a scale of 10 to 100 mg; a distillation in the Kugelrohr is then the end of the experiment.
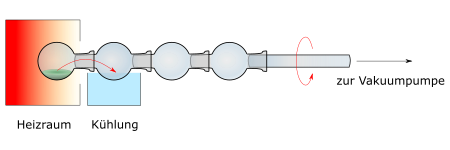
Ball tubes are made by glassblowers in various sizes; they consist of at least two balls. The substance to be distilled, mostly dissolved in a solvent, is brought into the ball at the end of the tube with an elongated funnel or a long pipette, whereby the inside of the glass tube and the other balls must not be wetted (contaminated). Alternatively, when using modern spherical tube apparatus, a spherical tube is used, which consists of several glass spheres with standard ground joints. The spheres are connected to the apparatus via a glass tube with a ground joint. The substance to be distilled is filled into the ball, which has a standard joint (boiling ball). Before the distillation begins, the (as low-boiling) solvent, z. B. diethyl ether or dichloromethane, removed by "applying a vacuum" while turning the bulb tube.
For distillation, the boiling sphere is positioned in an "oven", the temperature of which can be regulated. As soon as the boiling temperature is reached, the steam is caught in the second ball outside of the oven. This is cooled by contact with water, ice or dry ice. The spherical tube must be rotated constantly. This avoids boiling delays and the material to be distilled in the boiling sphere is distributed as a film on the inner wall; Thus, the distillation from the thin film takes place under gentle conditions. The Kugelrohr distillation can be classified under the so-called short path distillations.
After the distillation has ended, the bulb tube is taken out of the oven and allowed to cool to room temperature. The distilled substance can be removed from the ball with a curved pipette.
If there is a mixture of several volatile compounds, a ' fractional ' distillation can be achieved to a certain extent by pushing the second ball, filled with the first distillate, into the furnace and now cooling the third ball as a "receiver". Depending on the number of balls, this game can be repeated. The effectiveness of the separation of substances with similar boiling points (separation efficiency) is, however, quite low; In other words: the number of theoretical trays is small.
construction
Originally, the spherical tubes were turned back and forth by hand during distillation. Later, electric motors with special gears could take over the rotary motion. The equipment required for this was built in the workshops of the chemical institutes. Kugelrohr apparatus became generally available after manufacturers of laboratory equipment had taken on their development.
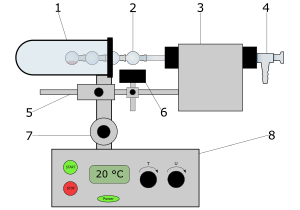
A modern Kugelrohr apparatus has an electrically heated and thermostated oven in which the first sphere is heated with hot air. The oven is closed off by a variable iris diaphragm behind the "boiling sphere" so that as little heat as possible is lost. The glass tube is inserted into a coupling via a sealing ring (O-ring), which is rotated by the regulated motor. A hose leads to the vacuum pump.
The distillate is cooled either with air, ice or dry ice .
Further use
A Kugelrohr oven can also be used for drying solids , for sublimation or for removing solvents or their residues in a vacuum.
Individual evidence
- ↑ Victor Regnault: Textbook of chemistry for universities, high schools, secondary and commercial schools, as well as for self-teaching. Volume 1, Duncker and Humblot, Berlin 1849, p. 142.
- ↑ Entry on Kugelrohr. In: Römpp Online . Georg Thieme Verlag, accessed on June 4, 2020.
- ↑ Kugelrohr apparatus at Sigma-Aldrich .
- ↑ Kugelrohr apparatus at Büchi .
literature
- HGE Loewenthal, E. Zass: The clever organic chemist. Guide to Success in Synthesis. JA Barth, Leipzig et al. 1993, ISBN 3-335-00360-8 , pp. 192-194.