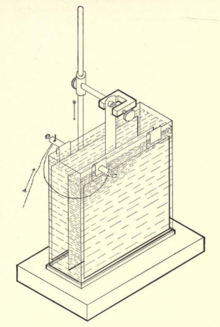
Copper coulometer
The copper coulometer is - like all coulometers - a historical device that was used to determine electrical charges and constant currents in the direct current circuit . It was widely used from around 1880 until after the middle of the 20th century. In particular, copper coulometers have been used for larger currents for which the more accurate silver coulometers of the appropriate size would have been very expensive. Like all coulometers, the copper coulometer no longer has any practical significance; it is only used for training purposes.
The copper coulometer contains at least two copper sheets or plates as electrodes , which are in a slightly acidic copper (II) sulfate solution. The flow of current through the series-connected cell dissolves copper on the positive copper electrode ( anode ) and copper is deposited from the solution on the copper cathode . The total amount of electrical charge transferred can be calculated from the change in mass of the dried copper electrodes determined with a scale . If the current is constant, it is calculated from the known electrolysis time.
The electrolyte mostly consisted of 150 g copper sulfate , 50 g sulfuric acid , 50 g ethanol and 1000 g water .
Historical
The copper coulometer, in the 19th century copper voltameter called, was in Germany no later than 1883 known in England no later than 1886. The above electrolyte composition was in 1893 recommended in an article by Felix Oettel; the electrolyte made of copper sulfate CuSO 4 , sulfuric acid H 2 SO 4 , ethanol C 2 H 5 OH and water H 2 O is therefore also called Oettel's solution .
variants
For more precise measurements, platinum sheet was also used as the cathode on which the copper was deposited. In particular with large currents it is advantageous to use a cathode plate made of stainless steel, since this can better carry the weight of the deposited copper, and since it is possible to pull the deposited copper off the steel and weigh it when folded.
Reaction equations
- Anode (positive pole, oxidation)
- Cathode (negative pole, reduction)
- Overall process
The total concentration of the solution and the total mass of copper do not change.
Charge calculation
According to Faraday's laws , the charge Q is proportional to the change in mass, and the following applies:
- Q : transferred electric charge
- Δ m : change in mass of the copper cathode, determined with the most accurate balance possible
- z : number of electrons transferred per particle. For Cu / Cu 2+ is z =. 2
- F : Faraday constant (≈ 96,485.3 As mol −1 )
- M : molar mass . For copper: M = 63.546 g / mol.
- Ä e : electrochemical equivalent , Ä e = M / z F . For copper: Ä e = 0.3293 mg / As.
Individual evidence
- ^ Gustav Kortüm: Textbook of electrochemistry . Verlag Chemie, Weinheim 1952.
- ↑ Uni Saarbrücken: Experiment instructions for the conductivity of electrolytes (PDF; 134 kB)
- ↑ Uni Basel: Module Electricity II, Faraday constant (PDF), accessed August 1, 2016.
- ^ A b AK Datta, N. Dhar: Accuracy of copper voltameters . In: Journal of the American Chemical Society . tape 38 , no. 6 , 1916, pp. 1156–1160 , doi : 10.1021 / ja02263a002 .
- ^ Hermann Hammerl: Study about the copper voltameter . In: Electriciens Berlin (ed.): Electrical magazine . tape 4 , no. 12 . Julius Springer, Berlin 1883 ( Internet Archive ).
- ^ Thomas Gray: On the Electrolysis of Silver and of Copper, and the Application of Electrolysis to the Standardizing of Electric Current and Potential meters . In: The London, Edinburgh and Dublin philosophical magazine and journal of science . tape 22 , no. 138 , 1886, p. 389-414 ( Internet Archive ).
- ^ Felix Oettel: Chemiker-Zeitung . tape 17 , 1893, pp. 543, 577 .
- ↑ Konrad North: Oettelsche solution. In: Viktor Engelhardt (Hrsg.): Electrolytic counter (= monographs on applied electrochemistry . Volume 31 ). Knapp, hall a. P. 1908, OCLC 248771234 , p. 37 ( delibra.bg.polsl.pl [PDF]).
- ↑ Felix Auerbach: Current measurement . In: Adolph August Winkelmann (Ed.): Handbuch der Physik . 2nd Edition. tape 4 . J. Barth, Leipzig 1905, p. 310 ( Internet Archive ).
- ↑ David Schlain, Charles B. Kenahan: A copper coulometer for use with large currents . In: Journal of Chemical Education . tape 35 , no. 3 , 1958, pp. 144 , doi : 10.1021 / ed035p144 .