Mixed heat
The heat of mixing h E (also referred to as mixing enthalpy or excess enthalpy ) is the heat that occurs when pure chemical substances are mixed :
- If the mixed heat is absorbed by the mixed substances from the environment , it is an endothermic course.
- If the mixed heat is given off by the mixed substances to the environment , it is an exothermic course
In this article, the symbol h E refers to the molar heat of mixing, i.e. H. the heat of mixing per amount of substance .
Examples
Depending on the mixing partner, the mixing of chloroform can be exothermic or endothermic (all examples for approx. 25 ° C.):
- Mixing with tetrahydrofuran is highly exothermic (about -2800 J / mol ).
- Mixing with ethanol takes place depending on the selected amount of source substance
- either exothermic (20 mol% chloroform and 80 mol% ethanol: about -650 J / mol)
- or endothermic (20 mol% ethanol and 80 mol% chloroform: approx. +400 J / mol)
- Mixing with cyclohexane is endothermic (approx. +700 J / mol).
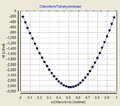
- Mixing enthalpies at T = 25 ° C
Modeling
Mixture heat curves of binary mixtures at a given temperature can be described with the equations according to Redlich-Kister (RK) and a sum of symmetrical functions (SSF). Both series developments are based on the following simple relationship, which, however, is only sufficiently precise for a few systems:
With
- , : Molar fractions of the two components
- : Constant.
Redlich-Kister
With
- A i : adjustable parameter
- n = 1..6 (one to six parameters).
Sum of symmetric functions
With
- A i , a i : adjustable parameters
- m = 1..3 (two, four or six parameters).
literature
- ↑ Excess Enthalpy Data. DDBST GmbH, accessed on March 16, 2017 (English).
- ↑ Christensen C., Gmehling J., Rassmussen P., Weidlich U., Holderbaum T., "Heats of Mixing Data Collection", DECHEMA Chemistry Data Series Vol. III., DECHEMA , Frankfurt / M., 1984-1991.
- ↑ Redlich O., Kister AT, "Algebraic Representation of Thermodynamic Properties and the Classification of Solutions", Ind.Eng.Chem., 40 (2), 345-348, 1948.
See also
- gE models
- Enthalpy of solution
- Activity coefficient
- The Dortmund database also contains mixed heats.