Mislow-Evans rearrangement
The Mislow-Evans rearrangement , also known as the Mislow-Evans reaction , is a name reaction in organic chemistry and was discovered in 1971 by the American chemists Kurt Mislow and David Evans . It is a reaction for the preparation of alkenols from alpha- substituted allyl sulfoxides .
Overview reaction
David Evans recognized that when α-substituted allyl sulfoxides are heated in the presence of thiophiles, alkenols are formed.
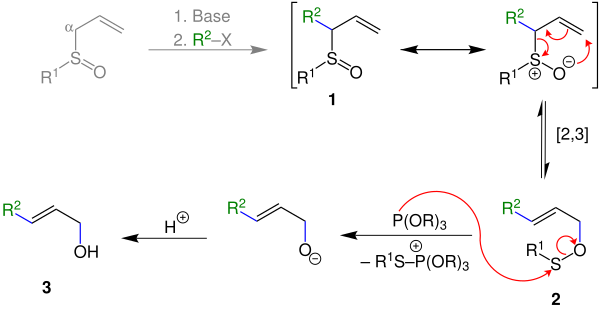
The sulfoxides 1 required for this can be synthesized from α-unsubstituted sulfoxides with the addition of a base and a haloalkane.
The actual reaction can be carried out with a wide variety of substituents. Alkyl and allyl groups are suitable for R 1 , and alkyl, aryl and benzyl groups for R 2 .
mechanism
A possible mechanism is given by László Kürti and Barbara Czakó.
Accordingly, the main reaction step is a sigmatropic [2,3] rearrangement :
An alpha-substituted allyl sulfoxide 1 can be prepared from an allyl sulfoxide with the addition of a base and a halogenated hydrocarbon . In the first reaction step of the Mislow-Evans rearrangement, this rearranges sigmatropically to ester 2 when heat is supplied . A thiophile is then added. This attacks the sulfur atom of ester 2 . An alcoholate ion is formed when a cation is split off. This alcoholate ion is protonated with the addition of acid to form the end product 3 , the alkenol.
application
The reaction is mainly used for the stereoselective synthesis of alkenols from allyl sulfoxides. This is used, among other things, in natural product synthesis. For example, Douglas Taber used the Mislow-Evans rearrangement to synthesize the hormone prostaglandin E2 , which is one of the most important hormones in the group of prostaglandins ( tissue hormones ).
Individual evidence
- ↑ a b c d László Kürti and Barbara Czakó .: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms , Elsevier Academic Press, 2005, pp. 292-293, ISBN 978-0-12-429785-2 .
- ↑ a b Tang, R., Mislow, K. Rates and equilibria in the interconversion of allylic sulfoxides and sulfenates. J. Am. Chem. Soc. 1970, 92, 2100-2104, doi: 10.1021 / ja00710a051 .
- ↑ a b Evans, DA, Andrews, GC, Sims, CL Reversible 1,3 transposition of sulfoxide and alcohol functions. Potential synthetic utility. J. Am. Chem. Soc. 1971, 93, 4956-4957, doi: 10.1021 / ja00748a075 .