Missing-self hypothesis
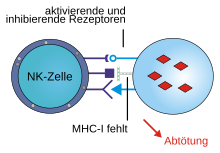
The missing-self hypothesis in immunology states that natural killer cells ( NK cells ) kill other endogenous cells if they do not have a certain signal protein on their surface . Corresponding signal proteins to prevent killing by the NK cells are therefore formed by almost all healthy body cells. NK cells are lymphocytes without receptors for specific antigens that are part of the innate part of the immune system .
Immunological background
The signal proteins that prevent the NK cells from being active against the body's own cells are MHC class I molecules . There are corresponding receptors on the surface of the NK cells, which the MHC molecules recognize. If more inhibitory receptors than activating ones interact with the MHC molecules, as is the case with healthy cells, the lysis of the target cell by the NK cell is suppressed. Among the most important receptors of NK cells include the for differentiation Cluster of belonging CD94 molecule and homologous proteins, as well as in humans, the "killer cell immunoglobulin like receptors" ( KIR ) and the mouse Ly49 proteins.
If there is a lack of corresponding MHC molecules on the surface of the body's own cells, for example in tumor cells or virus-infected cells, it can happen that more activating receptors interact with the MHC molecules and so the activating signal to the cell is greater than the inhibiting one. As a result, the target cell is killed by the NK cells. This is done by releasing perforins and granzymes . Perforins create openings in the membrane of the target cell through which the granzymes penetrate the cells. The granzymes are proteases that initiate apoptosis in the target cell by activating appropriate signal cascades , a form of programmed cell death . In addition, the NK cells trigger apoptosis by forming the so-called Fas ligand on their surface. Its binding to the Fas receptor on the surface of the target cells activates the corresponding apoptotic signaling pathways in them.
The missing-self hypothesis was proposed in the mid-1980s by Klas Kärre , currently professor at the Center for Microbiology and Tumor Biology at the Karolinska Institute in Sweden, based on the results of his doctoral thesis.
Individual evidence
- ↑ Klas Kärre: Role of Target Histocompatibility Antigens in Regulation of Natural Killer Activit. A reevaluation and a hypothesis. In: Ronald B. Herbermann, Denis M. Callewaert (Eds.): Mechanisms of NK Cell Mediated Cytotoxicity. Academic Press, Orlando 1985, ISBN 0-12-341370-2 , pp. 81-91.
literature
- K. Karre: NK cells, MHC class I molecules and the missing self. In: Scandinavian journal of immunology. Volume 55, Number 3, March 2002, pp. 221-228, PMID 11940227 (review).