Nickel test
A nickel test is used to detect nickel in metal objects, alloys and solutions.
Because some people are allergic to nickel, rapid nickel tests are available to check e.g. B. jewelry, eyeglass frames or other objects that come into contact with the skin to test for the presence of nickel.
A nickel test can also be used to prove the meteoritic origin of pieces of iron found.
Qualitative rapid tests
With the classic quick tests for the qualitative testing of jewelry etc. for nickel you get z. B. from mail order pharmacies a set consisting of prepared cotton swabs and a separate solution.
To test, the cotton swab is moistened with 1 - 2 drops of the solution and then rubbed the surface to be examined with the swab for about 30 seconds. If nickel is present or released from the surface of the abraded object, the cotton ball turns pink to red to varying degrees, depending on the amount of nickel.
However, these rods do not allow any direct conclusions to be drawn about the amount of nickel released or contained in the material.
Semi-quantitative and quantitative tests
Test sets are also available for a semi-quantitative analysis of the released nickel in the corresponding concentration ranges. The test sticks to be used here are dipped into the solution to be examined for the nickel content and, after a short waiting time, compared with a color scale that provides information about the concentration range of nickel contained in the solution.
These test sticks are also suitable for use on metallic solids, but then do not allow any conclusions to be drawn about the amount of nickel released and only provide a qualitative statement.
The quantitative determination of the nickel content cannot be reliably carried out / realized with test strips. There are also test sets / nickel tests for precise quantification, but the samples that have been treated with the set must always be transferred to a different, device-based analysis method and are therefore only used for sample preparation. As a rule, these are photometers .
Chemical background
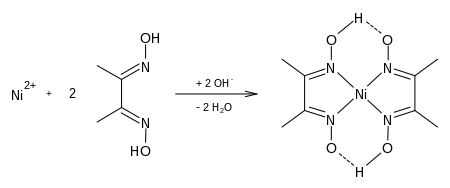
In an alkaline environment, in combination with dimethylglyoxime, nickel ions form a poorly soluble, intensely red-colored complex , bis (dimethylglyoximate) nickel (II):
For the quick test method, the cotton swabs were soaked in a solution of dimethylglyoxime by the manufacturer and dried again so that each swab contains an approximately defined amount of reagent. The solution to be applied to the stick consists predominantly of ethanol and a small amount of ammonia in order to set the pH value to be basic. If this stick is now rubbed over the surface to be tested, existing nickel ions pass into the solution and form the complex described here via the extremely sensitive reaction.
The rods for the semi-quantitative statement in a concentration range contain a much more precisely set amount of the reagent and can thus make statements about the amount of nickel contained in the respective solution examined.
The sets for the exact quantitative determination mostly contain further solutions with additional reagents for further sample pretreatment and / or preservation of taken samples that cannot be examined in immediate temporal relation.
Since iron (II) ions, for example, form a complex with a similar red color, but iron (III) ions do not, this is first converted into the trivalent oxidation state by adding oxidizing agents .
The detection limit is around 0.015 µg nickel / ml solution.
Individual evidence
- ↑ apo-rot mail order pharmacy: NICKEL test sensitive test sticks. Retrieved March 30, 2018 .
- ↑ Merck KGaA: 110006 | Nickel test. Retrieved March 30, 2018 .
- ↑ Merck KGaA: 114785 | Nickel test. Retrieved March 30, 2018 .
- ↑ a b c G. Jander, H. Wendt: Textbook of analytical and preparative inorganic chemistry . 2nd Edition. S. Hirzel Verlag, Leipzig 1954, p. 108 f .