Proton gradient
A proton gradient is a spatial or temporal difference in the concentration of protons . It is the difference in the concentration of protons, more precisely of hydrated H + ions (H 3 O + or oxonium ), per unit of distance or unit of time at a certain point in space. Since the pH as the logarithm of the H + is defined concentration, are proton gradient and pH - Gradient used synonymously.
Proton gradients can be extended over long distances (e.g. between bodies of water with different pH values) or over short distances (e.g. on biomembranes ). A very abrupt change in concentration is called a discontinuous gradient .
properties
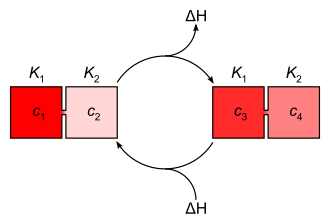
Each concentration gradient corresponds to an energy potential ΔH , which results from the difference between the solution enthalpies or the steepness of a proton concentration gradient and, in the case of proton gradients, also from the neutralization energy . When the concentration is equalized between the areas with different pH values, energy is released (exothermic reaction), which can be used through energetic coupling .
biochemistry
PH gradients can be measured on the membranes of living cells. According to the chemiosmotic theory , an electrochemical membrane potential follows from a proton gradient , which is used by chemiosmotic coupling .
Proton gradients are used in biochemical laboratory practice, among other things, in SDS-PAGE to concentrate proteins in a polyacrylamide gel , in isoelectric focusing , in centrifugation , in ion exchange chromatography and in affinity chromatography .
literature
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Biochemistry . 6th edition. Elsevier Spektrum Akademischer Verlag, Heidelberg 2007, ISBN 978-3-8274-1800-5
- David L. Nelson, Michael Cox: Lehninger Biochemistry . 3. Edition. Springer, Berlin a. a. O. 2001, ISBN 3-540-41813-X .
- Gerhard Gottschalk: Bacterial Metabolism . 2nd Edition. Springer, New York a. a. O. 1988. ISBN 3-540-96153-4 .
Individual evidence
- ^ John A. Baross, Sarah E. Hoffman: Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. In: Origins of Life and Evolution of the Biosphere. 15, 1985, p. 327, doi : 10.1007 / BF01808177 .
- ↑ MR Gunner, M. Amin, X. Zhu, J. Lu: Molecular mechanisms for generating transmembrane proton gradients. In: Biochimica et Biophysica Acta . Volume 1827, number 8-9, 2013 Aug-Sep, ISSN 0006-3002 , pp. 892-913, doi : 10.1016 / j.bbabio.2013.03.001 , PMID 23507617 , PMC 3714358 (free full text).
- ↑ Gerhard Richter: Practical Biochemistry: Basics and Techniques. Georg Thieme Verlag, 2003. ISBN 9783131323811 . P. 90.
- ^ Pier Giorgio Righetti: Immobilized pH Gradients: Theory and Methodology: Theory and Methodology. Laboratory Techniques in Biochemistry and Molecular Biology. Elsevier, 1990. ISBN 9780080858890 .
- ↑ W. Borchard, A. Straatmann: Analytical Ultracentrifugation VI. Volume 119 of Progress in Colloid and Polymer Science . Springer, 2003. ISBN 9783540446729 . P. 13.
- ↑ Shuichi Yamamoto, Kazahiro Nakanishi, Ryuichi Matsuno: Ion-Exchange Chromatography of Proteins . In: Chromatographic Science Series. CRC Press, 1988. ISBN 9780824779030 . P. 284.
- ^ Robert K. Scopes: Protein Purification: Principles and Practice. In: Springer Advanced Texts in Chemistry . Springer Science & Business Media, 1994. ISBN 9780387940724 . Pp. 154, 155.