Pfeffer's cell
The Pfeffer's cell is a device developed by the German botanist Wilhelm Pfeffer to measure the osmotic pressure of aqueous solutions . Its functional principle is that of a membrane osmometer . Pfeffer described them in his work Osmotic Investigations , published in 1877 .
Structure and mode of operation
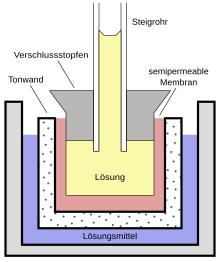
In the form originally developed by Pfeffer, the Pfeffer's cell consists of an unglazed clay vessel covered with a colloidal film. This film acts as a semi-permeable membrane that is good for water, but practically impermeable for dissolved substances such as sugar or salts . To create the film, Pfeffer dipped the clay vessel first in a solution of copper sulfate and then in dissolved potassium hexacyanidoferrate (II) (yellow blood liquor salt), which caused precipitation membranes of copper (II) hexacyanoferrate (II) to form in the pores of the vessel. Pfeffer also experimented with precipitation membranes made from other substances such as Prussian blue . Occasionally he used membranes made of calcium phosphate , iron phosphate or " iron oxide hydrate ", which are not decomposed by alkaline solutions .
The solution to be measured (for example a sugar solution) is filled into the prepared vessel, which is closed at one end with a stopper and then placed in a larger vessel with pure water. A glass riser pipe is stuck in the stopper. If water penetrates the cell from the outside through osmosis, the liquid in the riser pipe will rise until its hydrostatic pressure corresponds to the osmotic pressure of the originally filled solution (before it is diluted by the inflowing water). The pressure increase can either be determined from the height of the liquid column or measured directly using a manometer . Pfeffer used a two-legged air manometer attached to the clay cell as shown in Figure 1 .
meaning
Measurement of osmotic pressure
With the Pfeffer's cell it was possible for the first time to precisely measure the osmotic pressure . Before Pfeffer, Moritz Traube and other naturalists had experimented with glass tubes in which an opening was closed by a precipitation membrane and the solution to be examined was poured in. The tube was then immersed in the surrounding liquid. This arrangement was not mechanically stable, since the membrane could easily tear at the pressures that occurred. A quantitative determination of the osmotic pressure was not possible.
Other researchers soon picked up on Pfeffer's development: For example, the Dutch chemist Jakobus van 't Hoff developed his fundamental work on the analogy between vapor pressure and osmotic pressure on the basis of Pfeffer's measurement results . The American chemist Harmon Northrop Morse found an electrolytic method for applying the precipitation membranes and was thus able to confirm and improve van 't Hoff's theory. Today, the Pfeffer's cell with colloidal precipitation membranes is practically no longer used as an osmometer, as the production is associated with a great deal of effort and the "membrane formers" used (such as copper sulfate and yellow blood liquor salt) must be present in the solutions used.
Osmotic model of the plant cell
Pepper plant cells , in which the semipermeable plasma membrane rests on the mechanically stable cell wall as an abutment, served as a model for its development . In this analogy, the precipitation membrane corresponds to the plasma membrane and the clay cell to the cell wall.
Pfeffer carried out numerous measurements with his apparatus, with which he investigated the water flow and pressure conditions for differently concentrated solutions. His motivation was to “get to know the cause of the often very high hydrostatic pressure forces in plant cells” ( Osmotic examinations - 8th diosmosis of dissolved bodies , p. 47). For the discussion of the “cell mechanics” in the second part of the osmotic investigation , he used the Pfeffer's cell as a model for the osmotic behavior of living cells . The structure and properties of the plasma membrane were little researched in Pfeffer's time.
Today, in plant physiology, the Pfeffer's cell is still described, mostly in a modified form, to illustrate the osmometer and the water potential .
Web links
- Osmotic studies - studies of cell mechanics at www.archive.org
Individual evidence
- ^ A b Wilhelm Pfeffer: Osmotic investigations . Wilh. Engelmann, Leipzig 1921. (2nd, unchanged edition of the first print from 1877).
- ^ JH van 't Hoff: The role of osmotic pressure in the analogy between solution and gases. In: Journal of Physical Chemistry. 1, 1887, pp. 481-508 ( Uri Lachish website, PDF ).
- ^ Harmon Northrop Morse: The Osmotic Pressure of Aqueous Solutions: Report on Investigations Made in the Chemical Laboratory of the Johns Hopkins University During the Years 1899-1913 . Carnegie institution of Washington, 1914 ( online at www.archive.org ).
- ↑ P. Sitte , EW Weiler, JW Kadereit, A. Bresinsky, C. Körner: Strasburger - Textbook of Botany . Spectrum Gustav Fischer 2002, ISBN 3-8274-1010-X , chapter water potential .