Plancher rearrangement
The Plancher rearrangement - also Ciamician-Plancher rearrangement - is a name reaction from the field of organic chemistry , which was published in 1896 by the chemist Giuseppe Plancher and Giacomo Luigi Ciamician . The Plancher rearrangement describes the thermal rearrangement of an alkyl or aryl group of a disubstituted indolin -3-ol. This forms the thermodynamically more stable indole .
Overview
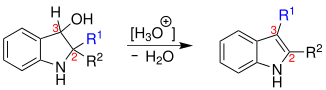
The indolin-3-ol, disubstituted at position 2 by alkyl or aryl groups, is converted to an indole by rearrangement of one of the alkyl / aryl groups ( blue ) from position 2 to position 3 with elimination of water :
However, the reaction only takes place with acid catalysis and the supply of heat.
Reaction mechanism
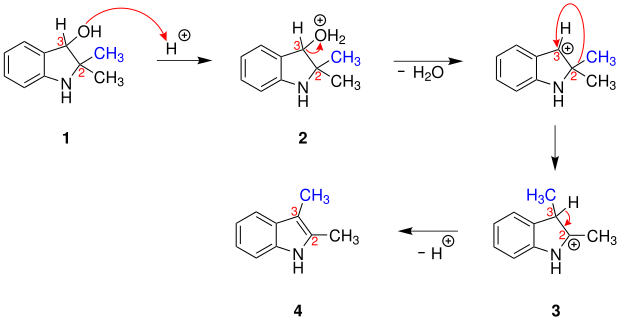
The following illustration describes a proposal for the reaction mechanism using the example of 2,2- dimethylindolin -3-ol:
First the hydroxyl group of 2,2-dimethylindolin-3-ol ( 1 ) is protonated and the (2,2-dimethylindolin-3-yl) oxonium ion ( 2 ) is formed. The oxonium group is then split off as a water molecule and the methyl group ( blue ) rearranged from position 2 to position 3. This creates the 2,3-dimethylindolium ion ( 3 ). Finally, a proton is split off at position 3, thus forming 2,3-dimethylindole ( 4 ).
Individual evidence
- ↑ G. Ciamician, G. Plancher, Ber. , 1896 , vol. 29, p. 2475.
- ^ B. Witkop, JB Patrick, J. Am. Chem. Soc. , 1951 , vol. 73, p. 1558.
- ↑ DJ Halett, U. Gerhard, SC Goodacre, L. Hitzel, TJ Sparey, S. Thomas, M. Roley, RG Ball, J. Org. Chem. , 2000 , Vol. 65, p. 4984.
- ↑ a b c d e Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents, 3 Volume Set , John Wiley & Sons, Hoboken, New Jersey, 2009 , ISBN 978-0-471-70450-8 , pp. 2248-2249.