Free radical polymerization with reversible deactivation
| This item has been on the quality assurance side of the editorial chemistry entered. This is done in order to bring the quality of the articles on the subject of chemistry in terms of form and content to the level desired in Wikipedia. We are grateful for your help , please take part in the discussion ( new entry ) or revise the article accordingly. |
Radical polymerization with reversible deactivation ( RDRP) belongs to the class of polymerization with reversible deactivation, which has a bit of the character of a “living polymerization”, but cannot be classified as such without chain growth or chain termination.
Different names can be found in the literature:
- Living radical polymerization
- Living free radical reaction
- Controlled / living radical polymerization
- Controlled radical polymerization
- Radical chain reaction with reversible deactivation
Although the term “living radical polymerization” was used earlier, it was not recommended by IUPAC because radical polymerization cannot really be a living process because of the inevitable chain termination reaction between two radicals . The commonly used expression “controlled radical chain reaction” is permitted, but “radical polymerization with reversible deactivation” or “controlled radical chain reaction with reversible deactivation” are recommended.
History and characteristics
RDRP - sometimes misleadingly referred to as "free" radical polymerization - has been one of the most widely used polymerization processes since it was first used:
- it can be carried out in the presence of certain functional groups
- the technology is simple and easy to control
- it is relatively inexpensive compared to other methods
- the reaction conditions can vary from solutions to emulsions to suspensions
The equilibrium concentration of a growing polymer chain is on the order of 10 −7 M and the average lifetime of a single polymer radical before chain termination is 5–10 s. The disadvantage of conventional radical polymerization is the limited control of the chain architecture and the variability in molecular weight and composition. In the late 20th century it was observed that after adding certain components (the radical chain carrier ) to the polymer systems, a temporarily "sleeping state" could be achieved.
This had an influence on the extension of the life of the growing polymer chain with values comparable to the duration of the experiment. Radicals are always in an inactive (sleeping) state, but not irreversibly destroyed. Only a small part of them are active (growing) because the rate of conversion from the inactive to the active state of a radical is faster than the reaction rate at which the chain growth takes place. There is the same growth rate for all chains, that is, the chains all grow on average at the same rate. Consequently, the normal law of probability , the Poisson distribution , applies to the degree of polymerization for polymer chains , and a low dissolution rate predominates.
IUPAC also recognizes the alternative name "controlled reversible deactivation radical polymerization" as acceptable, provided that "controlled" is specified in the context, which means that in that case the molecular mass and the molecular mass distribution are taken into account. These types of radical polymerizations are not "living" polymerizations because chain termination reactions are not excluded.
The term "controlled" means either that the kinetics of the reaction are controlled, or that a particular polymeric product is formed, or both. The term "controlled reaction" is sometimes used to describe a free radical polymerization or an ionic polymerization, the reversible deactivation of the chain carrier being an essential component of the mechanism that disrupts chain propagation and affects the reaction kinetics of the polymerization as well as the structural aspects of the macromolecules that form, or both. The term "controlled radical polymerization" is sometimes used to describe a radical polymerization that occurs by a particular mechanism, such as atom transfer radical polymerization (ATRP) or reversible addition fragmentation chain transfer polymerization (RAFT). All of these and other controlled polymerization reactions belong to the class of reversible deactivation radical reactions.
Radical polymerization with reversible deactivation in comparison
There is a type of radical polymerization with reversible deactivation which is different from living polymerization except in the general characteristics. "Living polymerization" requires the complete absence of termination reactions, whereas radical polymerization with reversible deactivation involves a termination reaction similar to conventional polymerization with the same concentration of active species. The following table compares the essential aspects:
| Standard radical polymerization | Living polymerization | Free radical polymerization with reversible deactivation | |
|---|---|---|---|
| Concentration of the initial starter | only slowly decreases | decreases rapidly | decreases rapidly |
| Concentration of the chain carrier (Number of growing chains) |
decreases during the reaction | constant throughout the reaction | constant throughout the reaction |
| Life of the growing chain | same duration as overall reaction | same duration as overall reaction | |
| Type of termination reaction | Radical combination or radical disproportionation |
no irreversible chain termination | Radicals are reversibly deactivated / chain termination cannot be ruled out |
| Distribution of molar mass | broad distribution (Ð> = 1.5), Schulz-Zimm distribution |
narrow distribution (Ð <1.5), Poisson distribution |
narrow distribution (Ð <1.5), Poisson distribution |
| "sleeping" states | no | few | predominantly |
General properties
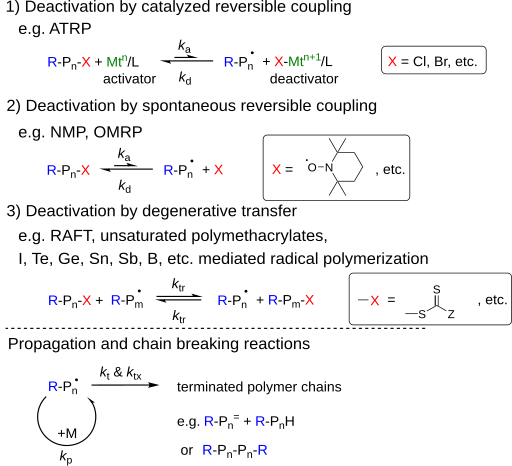
As the name suggests, the benefit of successful free radical polymerization is rapid and reversible activation / deactivation of the propagating chain. There are three types:
- Deactivation by catalyzed reversible coupling
- Deactivation by spontaneous reversible coupling
- Deactivation through degenerative transfer (DT)
A mixture of the different methods is possible. For example: a transition metal-controlled RDRP alternates between ATRP, OMRP and DT, depending on the reaction conditions and the reagents used.
In an RDRP reaction, the radicals can react with a reaction rate k p with the addition of a few monomers before deactivation occurs, which creates a "dormant" state. Two radicals can react with each other and generate unreactive chains with the reaction coefficient k t . The rate of chain propagation and the chain termination reaction between two radicals is not influenced by the mechanism of deactivation or by the catalyst used . So can one estimate how the reaction rate of the RDRP is influenced by the chain and its functional groups?
In addition, there are other chain termination reactions such as irreversible chain transfer or chain termination reactions of the migrating radical with the solvent, the monomer, the polymer, the catalyst, the additives ... which means an additional loss of chain end functionality. The total reaction rate of the chain termination reactions in addition to the direct reaction of two radicals is referred to as k tx .
In all RDRP methods, the theoretical number of the average molecular weight of the resulting polymers M n can be described by the following equation:
where M m is the molecular weight of the monomer; [M] 0 and [M] t are the monomer concentrations at time 0 and at time t ; [RX] 0 is the initial concentration of the initiator (radical starter).
In addition to a certain molecular weight, a well-controlled RDRP reaction can result in polymers with a narrow molecular distribution, which can be expressed in M w / M n values, and well-predetermined chains and functionalities.
A controlled RDRP reaction requires: 1) the process of reversible deactivation must be sufficiently fast, 2) the number of chain-breaking reactions that result in loss of chain and functionality should be limited, 3) the radical concentration must be maintained, and 4) the Free radical starters should have the appropriate activity.
Examples
Radical Atom Transfer Polymerization (ATRP)
The radical initiator of the polymerization is usually an alkyl or aryl halide and the "dormant" state is achieved by a metal complex of a transition metal . This method can be used universally, but requires unusual free- radical initiator systems that are poorly compatible with the polymerization medium.
Nitric Oxide Controlled Polymerization (NMP)
Under certain reaction conditions , a homolytic cleavage of the CO bond of an alkoxylamine can take place, so that a stable 2-electron-3-center bond is formed, which is able to start a polymerization reaction. The precondition for an alkoxyamine that is capable of initiating a polymerization is a secondary amine with large, sterically hindering substituents and a substituent on the oxygen atom which enables a stable radical to be formed, or benzyl.
Reversible addition-fragmentation chain transfer (RAFT)
RAFT is the most common and universally applicable technology in this context. Most RAFT processes are carried out in the presence of thiocarbonyl compounds , which act as radical buffers. While with the ATRP and NMP technology a reversible deactivation of the progressive radical-radical reaction takes place and "dormant" structures consist of an alkyl / aryl halide or an alkoxyamine with the NMP, which are both radical source and radical scavenger, and through describe an equilibrium reaction, RAFT, on the other hand, is a technique that is controlled by chain transfer reactions that are in an activation / deactivation equilibrium. Since no radicals are generated or destroyed in the reaction, an external radical source is required as a radical initiator for the reaction to proceed.
- Radical initiation in RAFT polymerization
- Reversible chain transfer

- Catching the radical
- Chain equilibrium reaction

- The End
Catalytic chain transfer and cobalt-controlled radical polymerization
Although strictly speaking it is not a living polymerization, catalytic chain transfer polymerization must be mentioned in this context because it illustrates the development of the later living free radical polymerization.
It was developed in the late 1970s when it was discovered that cobalt porphyrins are able to lose molecular weight during the polymerization of methyl acrylates. Later research showed that cobalt-glyoxime complexes are just as effective as porphyrin catalysts and also less sensitive to oxygen. Because of their lower sensitivity to oxygen, these catalysts have been studied more carefully than porphyrin catalysts.
The main products of catalytic chain transfer polymerization are vinyl terminated polymer chains. One of the major disadvantages of the process is that chain transfer catalytic polymerizations do not produce macro- monomers , but instead produce addition-fragmentation reagents. As a growing polymer chain reacts with an addition fragment, the radical end group attacks the vinyl group and forms a new bond. However, the resulting product is sterically hindered, so that the resulting species is subject to fragmentation that leads to a telechelic polymer.
While high chemical yields of macro-monomers are possible with methacrylates, relatively low yields are obtained when using catalytic chain transfer reagents during the polymerization of acrylates and styrene monomers. This is due to the interaction between the radical center and the catalyst during the polymerization reaction. The reversible reaction of the cobalt macrocycle with growing radical chains is known as the cobalt-carbon bond and in some cases leads to living polymerizations.
Iniferter Polymerization
An "iniferter" is a chemical compound that simultaneously acts as a radical starter , transfer reagent and terminator in controlled free radical infer -ter reactions, hence the name, with the dithiocarbamate type being the most common.
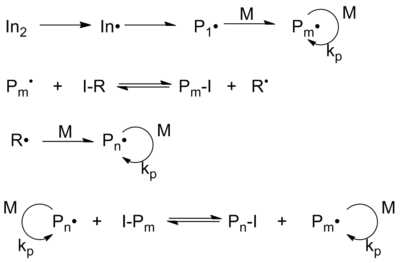
Iodine transfer polymerization (ITP)
Iodine transfer polymerization (ITP) was developed by Tatemoto and co-workers in the 1970s and produces fluoroolefin polymers. While little scientific attention is paid to the reaction, this chemistry is the basis for a number of industrial patents and products and may be the most commercially successful form of living radical polymerization. It was initially used to incorporate iodine healing agents into fluoroelastomers.
The ITP mechanism requires thermal decomposition of the free radical initiator (typically persulfate ), which generates the initial radical In •. The monomer M is added to this radical and thus creates the species P 1 •, which can propagate further to P m •. By exchanging the iodine from the transfer reagent RI to the migrating radical P m • the new radical R • is formed and P m • becomes a "dormant" radical. This species can react with the monomer M to form P n •. During the polymerization there is an exchange between different polymer chains and a transfer reagent is created, which is typical for degenerative transfer processes.
Typically, a mono- or diiodo-perfluoroalkane is used as the starting reagent in iodine transfer polymerization. This fluoroalkane may be partially substituted with hydrogen or chlorine. The energy of the iodine-perfluoroalkane bond is low and, in contrast to iodine-hydrogen bonds, their polarization is low.
Because of this, the iodine is easily split off in the presence of free radicals. If one considers iodoperfluoroalkanes, the iodine is split off as the polyfluoroolefin chain grows and the chain reaction is terminated, with the newly formed perfluoroalkyl radical attaching to another monomer. But the polyfluoroolefin with the iodine end itself acts as a chain transfer reagent. In a RAFT reaction, as long as the concentration of free radical initiator remains low, the product is a polymer with a monodisperse statistical distribution of the molecular weight.
Conventional hydrocarbon monomers with an iodoperfluoroalkane as a chain transfer reagent have already been reported. The resulting statistical distribution of molecular weight is not particularly narrow since the energies of the iodine-hydrogen bond are quite different from those of the iodine-fluorine-carbon bond and the removal of an iodine atom from the final polymer is correspondingly difficult. The use of iodine hydrocarbons has also been described, but again there was no narrow molecular weight distribution.
In addition, the preparation of block copolymers with iodine transfer polymerization by Tatemoto et al. reported.
Although the application of living radical reaction processes in emulsions is shown to be difficult, all examples of iodine transfer polymerizations were carried out in emulsions. Extremely high molecular weights are reported.
Other living radical polymerization techniques that are becoming increasingly important are described below.
Selenium-centered controlled radical polymerization
Diphenyl diselenides and some benzyl selenides have been reported by Kwon et al. checked for their effect as photoiniferters in polymerizations of styrene and methyl methacrylate. The proposed mechanism for this polymerization is similar to that of the dithiuam disulfide inifer. Low reaction constants allow application to block copolymer synthesis, but with limited control over the statistical distribution of molecular weight.
Telluride-controlled polymerization
Telluride -controlled polymerization, or TERP, initially appeared to proceed according to a reversible chain transfer mechanism with homolytic cleavage under thermal reaction conditions. However, a kinetic study has shown that TERP proceeds through degenerative transfer rather than a dissociation-combination mechanism.
Alkyl tellurides of structure ZXR, where Z = methyl and R = good free radical leaving group, give better controlled reactions for a wide range of monomers, while phenyl tellurides have little kinetic control. Polymerizations of methyl methacrylates take place in a controlled manner only with ditellurides. The importance of X for chain transfer increases in the series O <S <Se <Te, which makes alkyl tellurides effective under thermal initialization to carry out a controlled reaction, while for alkyl selenides and sulfides photochemical cleavage is necessary for radical initiation.
Stiban (antimony hydrogen) - controlled polymerization
Yamago et al. reported on a stibane-controlled polymerization in which an organostibane transfer reagent with the general structure Z (Z ') - Sb-R (where Z = activating group and R = free radical as leaving group) is used. A wide range of monomers (styrene, acrylates, vinyl ...) can react in a controlled manner, resulting in a narrow statistical distribution of molecular weight and predictable molecular weight under thermally induced reaction conditions. Yamago has also published a patent in which he proved that bismuth alkyls are subject to controlled radical polymerizations with a similar mechanism.
Copper - controlled polymerization
In addition, radical polymerizations with reversible deactivation under copper catalysis are known.
Individual evidence
- ↑ a b c Jenkins AD, Jones RG, Moad G: Terminology for reversible-deactivation radical polymerization previously called "controlled" radical or "living" radical polymerization (IUPAC Recommendations 2010) . In: Pure and Applied Chemistry . tape 82 , no. 2 , 2009, ISSN 1365-3075 , p. 483-491 , doi : 10.1351 / PAC-REP-08-04-03 .
- ↑ a b Szwarz, M .: 'Living' Polymers . In: Nature . tape 178 , no. 1 , 1956, pp. 1168-1169 , doi : 10.1038 / 1781168a0 .
- ↑ a b Szwarz, M .: Comments on "Living Polymerization: Rationale for Uniform Terminology" by Darling et al . In: J. Polym. Sci. A . tape 38 , no. 10 , 2000, pp. 1710 .
- ↑ Patent US4581429 : Inventors: DH Solomon, E. Rizzardo, P. Cacioli.
- ↑ G. Moad, E. Rizzardo: Alkoxyamine-Initiated Living Radical Polymerization: Factors Affecting Alkoxyamine Homolysis Rates . In: Macromolecules . tape 28 , no. 26 , 1995, pp. 8722-8728 , doi : 10.1021 / MA00130A003 .
- ↑ Zhong, M, Matyjaszewski, K: How Fast Can a CRP Be Conducted with Preserved Chain End Functionality? In: Macromolecules . tape 44 , no. 8 , 2011, p. 2668-2677 , doi : 10.1021 / ma102834s .
- ↑ Wang, Y, Soerensen, N, Zhong, M, Schroeder, H, Buback, Matyjaszewski, K: Improving the "Livingness" of ATRP by Reducing Cu Catalyst Concentration . In: Macromolecules . tape 46 , no. 3 , 2013, p. 683-691 , doi : 10.1021 / ma3024393 .
- ↑ Role of initiator-transfer agent-terminator (iniferter) in radical polymerizations: Polymer design by organic disulfides as iniferters Die Makromolekulare Chemie, Rapid Communications Volume 3, Issue 2, Date: 16 February 1982, pp. 127–132 doi: 10.1002 / marc.1982.030030208
- ↑ A model for living radical polymerization Die Makromolekulare Chemie, Rapid Communications Volume 3, Issue 2, Date: 16 February 1982, pp. 133-140 Takayuki Otsu, Masatoshi Yoshida, Toshinori Tazaki doi: 10.1002 / marc.1982.030030209
- ↑ Tatemoto, Masayoshi; Suzuki, Takeshi; Tomoda, Masayasu; Furukawa, Yasuyoshi and Ueta, Yutaka (1981-01-06) "Cross linkable fluorine-containing polymer and its production" patent US4243770 .
- ↑ B. Ameduri & B. Boutevin: Use of telechelic fluorinated diiodides to obtain well-defined fluoropolymers . In: J. Fluorine Chem. Volume 100 , 1999, pp. 97-116 , doi : 10.1016 / s0022-1139 (99) 00220-1 .
- ^ Carlson, Dana P. (1991-08-06) "Base resistant fluoroelastomers with improved processibility and curability". Patent US5037921 .
- ↑ Arcella, Vincenzo; Brinati, Giulio; Albano, Margherita and Tortelli, Vito (1996-12-17) "Fluoroelastomers comprising monomeric units deriving from a bis-olefin". Patent US5585449 .
- ↑ J. Banus, HJ Emeleus, RN Haszeldine: 12. The heterolytic fission of the carbon? Iodine bond in trifluoroiodomethane . In: J. Chem. Soc. 1951, p. 60 , doi : 10.1039 / jr9510000060 .
- ↑ M. Lansalot, C. Farcet, B. Charleux, J.-P. Vairon: Controlled Free-Radical Miniemulsion Polymerization of Styrene Using Degenerative Transfer . In: Macromolecules . tape 32 , no. 22 , 1999, p. 7354-7360 , doi : 10.1021 / ma990447w .
- ↑ K. Matyjaszewski, B. Gaynor, J.-S. Wang: Controlled Radical Polymerizations: The Use of Alkyl Iodides in Degenerative Transfer . In: Macromolecules . tape 28 , no. 6 , 1995, pp. 2093-2095 , doi : 10.1021 / ma00110a050 .
- ↑ K. Ziegler: The importance of the organic alkali metal compounds for synthesis . In: Angew. Chem. Band 49 , no. 30 , 1936, pp. 499-502 , doi : 10.1002 / anie.19360493003 .
- ↑ Tae Seok Kwon, Sadanori Kumazawa, Tetsuya Yokoi, Shuji Kondo, Hideo Kunisada & Yasuo Yuki: Living Radical Polymerization of Styrene with Diphenyl Diselenide as a Photoiniferter. Synthesis of Polystyrene with Carbon-Carbon Double Bonds at Both Chain Ends . In: Journal of Macromolecular Science, Part A . tape 34 , no. 9 , 1997, pp. 1553-1567 , doi : 10.1080 / 10601329708010026 .
- ↑ Goto A, Kwak Y, Fukuda T, Yamago S, Iida K, Nakajima M, Yoshida J: Mechanism-based invention of high-speed living radical polymerization using organotellurium compounds and azo-initiators . In: J. Am. Chem. Soc. tape 125 , no. 29 , 2003, p. 8720–1 , doi : 10.1021 / ja035464m .
- ↑ Yamago S, Ray B, Iida K, Yoshida J, Tada T, Yoshizawa K, Kwak Y, Goto A, Fukuda T: Highly versatile organostibine mediators for living radical polymerization . In: J. Am. Chem. Soc. tape 126 , no. 43 , 2004, p. 13908-9 , doi : 10.1021 / ja044787v .
- ↑ Yamago S, Kayahara E, Kotani M, Ray B, Kwak Y, Goto A, Fukuda T: Highly controlled living radical polymerization through dual activation of organobismuthines . In: Angew. Chem. Int. Ed. Engl. Volume 46 , no. 8 , 2007, p. 1304-6 , doi : 10.1002 / anie.200604473 .
![{\ displaystyle M _ {\ text {n}} = M _ {\ text {m}} \ times {\ frac {[{\ text {M}}] _ {0} - [{\ text {M}}] _ {t}} {[{\ text {RX}}] _ {0}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bafad896778ae585d925fb1cd6f62bc55cd633b6)