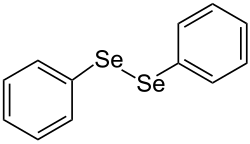
Diphenyl diselenide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diphenyl diselenide | |||||||||||||||
| other names |
Phenyl diselenide |
|||||||||||||||
| Molecular formula | C 12 H 10 Se 2 | |||||||||||||||
| Brief description |
yellow crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 312.13 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.557 g cm −3 |
|||||||||||||||
| Melting point |
63 ° C |
|||||||||||||||
| boiling point |
202 ° C |
|||||||||||||||
| solubility |
soluble in many organic solvents, insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Diphenyl diselenide is a chemical compound with the empirical formula (C 6 H 5 ) 2 Se 2 , also abbreviated as Ph 2 Se 2 . The orange solid is an oxidized derivative of selenophenol . It is used in organic synthesis to introduce PhSe units.
presentation
Diphenyl diselenide is obtained by reacting the Grignard reagent phenylmagnesium bromide (PhMgBr) with selenium under heating and subsequent oxidation by bromine (Br):
properties
Diphenyl diselenide has a centrosymmetric structure with an Se-Se bond length of 2.29 A.
Reactions
Characteristic reactions of diphenyl diselenide are reduction and chlorination:
Phenyl sodium selenide (PhSeNa) is a useful nucleophile that can be used to introduce a phenyl selenyl group by nucleophilic substitution of an alkyl halide , a mesyl group , a tosyl group, or an epoxide . The following example comes from morphine synthesis:
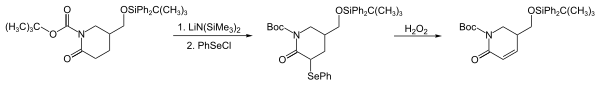
Phenyl selenium chloride (PhSeCl) is a powerful electrophile that can be used to introduce phenylselenyl groups into numerous nucleophiles such as enolates , enol silyl ethers , Grignard compounds, organolithium compounds , alkenes, and amines . In the reaction shown below (early reaction steps in the synthesis of strychnofoline), a phenylselenyl group is introduced by the reaction of PhSeCl with a lactom enolate . The reaction sequence is a powerful method for converting carbonyl compounds into their α, β-unsaturated analogues.
Diphenyl diselenide itself is also a source of the weakly electrophilic phenylselenyl group, but it can only be used in reaction with strong nucleophiles such as Grignard reagents, lithium compounds and enol esters. PhSeCl is both more reactive and efficient, since with Ph 2 Se 2 half of the selenium is wasted.
N- phenylselenophthalimide (N-PSP) can be used if PhSeCl is too strong and diphenyl diselenide is too wasteful.
Individual evidence
- ^ A b R. E. Marsh: The crystal structure of diphenyl diselenide . In: Acta Crystallographica . tape 5 , no. 4 , July 2, 1952, p. 458-462 , doi : 10.1107 / S0365110X52001349 .
- ↑ a b c Entry on diphenyl diselenide. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
- ^ Entry on diphenyl diselenide at ChemicalBook , accessed on February 10, 2013.
- ↑ a b c Datasheet Diphenyl diselenide, 98% from Sigma-Aldrich , accessed on February 11, 2013 ( PDF ).
- ↑ Hans J. Reich, Martin L. Cohen, Peter S. Clark: Reagents for Synthesis of Organoselenium Compounds: Diphenyl Diselenide and Benzeneselenenyl Chloride In: Organic Syntheses . 59, 1979, p. 141, doi : 10.15227 / orgsyn.059.0141 ; Coll. Vol. 6, 1988, p. 533 ( PDF ).
- ^ Douglass F. Taber, Timothy D. Neubert, Arnold L. Rheingold: Synthesis of ( -) - Morphine . In: Journal of the American Chemical Society . tape 124 , no. 42 , October 2002, p. 12416-12417 , doi : 10.1021 / ja027882h .
- ↑ Lerchner, A .; Carreira, EM: First Total Synthesis of (±) -Strychnofoline via a Highly Selective Ring-Expansion Reaction . In: Journal of the American Chemical Society . Vol. 124, No. 50 , 2002, pp. 14826-14827 , doi : 10.1021 / ja027906k .
- ^ Reich, HJ; Wollowitz, S .: Preparation of α, β- Unsaturated Carbonyl Compounds and Nitriles by Selenoxide Elimination . In: Organic Reactions . Vol. 44, 1993, pp. 1–296 , doi : 10.1002 / 0471264180.or044.01 .
- ↑ Barrero AF, Alvarez-Manzaneda, EJ; Chahboun, R .; Corttés, M .; Armstrong, V .: Synthesis and Antitumor Activity of Puupehedione and Related Compounds . In: Tetrahedron . Vol. 55, No. 52 , 1999, p. 15181-15208 , doi : 10.1016 / S0040-4020 (99) 00992-8 .