Contact angle
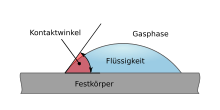
The contact angle ( theta ; also dihedral, edge or wetting angle ) is the angle that a drop of liquid on the surface of a solid forms with this surface.
meaning
From the measurement of the contact angle, e.g. B. with a contact angle goniometer , certain properties of the surface of a solid can be determined, z. B. the surface energy .
The size of the contact angle between liquid and solid depends on the interaction between the substances at the contact surface: the smaller this interaction, the larger the contact angle.
In the special case of using water as a liquid, the surface is called:
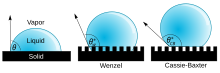
- at low contact angles (approx. 0 °, Figure 3a) as hydrophilic ("water-loving")
- at angles of 90 ° (Fig.3b) as hydrophobic (water-repellent)
- at angles over 90 ° (Fig. 3c) as superhydrophobic. The latter is also known as the lotus effect at very high angles (approx. 160 °) and corresponds to extremely low wettability .
The contact angle can be changed by surface treatment . In certain forms of surface structuring, omniphobic properties arise that are both hydrophobic and lipophobic . The opposite is amphiphilia .
theory
Young's equation

In 1805 defined Thomas Young contact angle of gas surrounding fluids on a solid surface as the angle at the phase boundary of the gaseous, liquid and solid phases ( Young's equation ):
with the interfacial tensions
- between solid and gaseous
- between solid and liquid
- between liquid and gas.
For finely structured surfaces
In the case of finely structured surfaces, R. Wenzel observed the change in the contact angle to :
with r as the ratio of the actual to the projected area.
The fine structuring strengthens the already existing properties of the surface: a hydrophobic surface (i.e. with a contact angle over 90 °) becomes even more water-repellent, e.g. B. the lotus effect, while a hydrophilic surface becomes even more hydrophilic.
Cassie and Baxter observed the change in the contact angle when the drop only rests on the bumps on the finely structured surface:
with as a contact surface between solid and liquid.
The following inequality must be fulfilled:
Alternative criteria for the Cassie-Baxter state are: The contact line forces must exceed gravity , and the fine structures must be long enough to prevent bridges from forming to the base. The contact angle has a hysteresis that is determined by the heterogeneity of the surface.
A model for predicting the surface properties uses the contact line density Λ, with four contact points Λ = 4x / y 2 .
The critical contact line density Λ c is described by the following equation:
With
- ρ = density of the liquid
- g = gravity
- V = volume of the liquid
- θ a = last contact angle before a movement
- θ a, 0 = last contact angle before moving on a smooth surface
- γ = interfacial tension of the liquid
- w = tower wall angle
If Λ> Λ c , the liquid is in the Cassie-Baxter state, otherwise in the Wenzel state. The changed last contact angle before a movement is described by the following equation:
with the Wenzel state:
With
- λ p = linear portion of the contact line to the unevenness
- θ r, 0 = returning contact angle on a smooth surface
- θ gas = contact angle between liquid and air (assumed to be 180 °)
attempt
The contact angle was measured on a coated silicon carbide pan. The pan bottom and the pan rim were in direct contact during the measurement. The measurements were carried out at the Technical University of Cologne on the Gummersbach campus (Institute for Materials Science and Applied Mathematics).
| Try with 1 drop -
Pan bottom |
Try with 1 drop -
Pan rim |
Try with 5 drops -
Pan bottom |
Try with 5 drops -
Pan rim |
|---|---|---|---|

|

|

|

|
See also
Web links
- Literature on contact angle and contact angle measurement
- Theoretical principles of contact angle measurement
Individual evidence
- ^ TL Liu, CJ Kim: Repellent surfaces. Turning a surface superrepellent even to completely wetting liquids. In: Science. Volume 346, Number 6213, November 2014, pp. 1096-1100, ISSN 1095-9203 . doi: 10.1126 / science.1254787 . PMID 25430765 .
- ^ T. Young: An Essay on the Cohesion of Fluids . In: Phil. Trans. R. Soc. Lond. . 95, 1805, pp. 65-87. doi : 10.1098 / rstl.1805.0005 . ( Full text )
- ^ RN Wenzel: Resistance of Solid Surfaces to Wetting by Water . In: Ind. Eng. Chem. . 28, No. 8, 1936, pp. 988-994. doi : 10.1021 / ie50320a024 .
- ↑ Pierre-Gilles de Gennes: Capillarity and Wetting Phenomena 2004, ISBN 0-387-00592-7 .
- ^ ABD Cassie, S. Baxter: Wettability of Porous Surfaces . In: Trans. Faraday Soc. . 40, 1944, pp. 546-551. doi : 10.1039 / tf9444000546 .
- ^ D Quere: Non-sticking drops . In: Reports on Progress in Physics . 68, No. 11, 2005, pp. 2495-2532. bibcode : 2005RPPh ... 68.2495Q . doi : 10.1088 / 0034-4885 / 68/11 / R01 .
- ↑ C Extrand: Criteria for Ultralyophobic Surfaces . In: Langmuir . 20, 2004, pp. 5013-5018.
- ^ RE Johnson, Robert H. Dettre: Contact Angle Hysteresis . In: J. Phys. Chem. . 68, No. 7, 1964, pp. 1744-1750. doi : 10.1021 / j100789a012 .
- ↑ C Extrand: Model for contact angles and hysteresis on rough and ultraphobic surfaces . In: Langmuir . 18, No. 21, 2002, pp. 7991-7999. doi : 10.1021 / la025769z .











![{\ displaystyle \ cos {\ theta} _ {CB} * = \ phi \ cdot [\ cos ({\ theta}) + 1] -1}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8e0611a26a2f69d91457f5522be4984fe8fb8b90)






