σ-bishomoaromaticity
σ-bishomoaromaticity is an extreme case of chemical bonding and aromaticity. It was first used as part of the Pagodan route for the synthesis of dodecahedrane by Prinzbach et al. described. In the case of σ-bishomoaromaticity, cyclic electron delocalization takes place within the plane defined by the atoms involved (and not in a π system aligned perpendicular to this plane, cf. benzene), the bond structure defining the perimeter being interrupted twice. The necessary conjugation takes place at these two points through space.
Background I: “classical” and σ-aromaticity
In organic chemistry, the phenomenon of aromaticity is of fundamental importance: the archetypal example of benzene shows that the cyclic conjugation of 4n + 2 π electrons (Hückel rule) leads to a significant increase in stability and thus significantly changed reactivity. In addition to the increased stability, the cyclic delocalization of the electrons is also responsible for a diamagnetic ring current, which can be detected by NMR measurements, as well as for an alignment of the bond lengths along the conjugated perimeter (cf. the equivalent C — C bond lengths in benzene compared to the alternation of CC single and C = C double bond lengths in the hypothetical cyclohexatriene).
If the cyclic delocalization does not take place in a π system, but rather through the interaction of σ bonds in the plane, one speaks of σ aromaticity. σ-aromaticity is linked to strict geometric boundary conditions and is found in the case of cyclopropane : its magnetic properties indicate a diamagnetic ring current, its comparatively low tension energy compared to cyclobutane (120.17 vs. 111.79 kJ / mol ) is due to stabilization intrinsically stronger CH bonds (net effect 33.5 kJ / mol) are attributed to aromatic stabilization through σ-aromaticity of 47.3 kJ / mol. and intrinsic binding energies .
Background II: homoaromaticity
In the classical case, aromaticity is achieved through cyclic electron delocalization in a π system that is defined by a continuous perimeter defined by σ bonds. If this σ perimeter is interrupted at one point, the interaction of the relevant π orbitals can still take place at this point with a favorable distance and orientation. In this case one speaks of homoconjugation, which takes place “through space” and leads to homoaromaticity in suitable systems . Typical examples can be found in the homotropylium cation or in cycloheptatriene .
Examples of σ-bishomoaromaticity
If, under suitable conditions (enforcement of the necessary geometrical arrangement by rigid cage systems), two CC double bonds overlap σ-like, the π orbitals form a closed perimeter in the σ plane, which allows cyclic electron delocalization. The associated orbitals are shown in the diagram opposite, the energetic position and order of the b 1 and b 2 orbitals depending on the distance between the two double bonds.
Such an arrangement of two double bonds is realized in the bisseco dodecahedron radii (see illustration).
-

- Fig .: Oxidation of pagodan and bisseco-dodecahedradien to sigma-bishomoaromatic 4-center-2-electron dication. Distances in Angstrom (X-ray structure for Pagodan, HF / 3-21G calculation for the diene and the dication)
If the σ-bishomoconjugate orbital system is occupied with 2 electrons (access by oxidation of the neutral species, only the energetically lowest a 1 orbital is occupied), one obtains astonishingly persistent 4-center-2-electron dications (4C / 2e), their high persistence u. a. is attributed to σ-bishomoaromaticity. Instead of the neutral precursors with the σ-overlapping CC double bonds, pagodan with its central cyclobutane ring can also be used. The reduction of the corresponding neutral species to the dianion, a 4-center-6-electron σ-bishomoaromatic, is of great scientific interest (Hückel rule), but has not yet been achieved with the carbon compounds. This was only achieved when the CC double bonds were exchanged for NN double bonds (bis-diazenes, see figure).
-
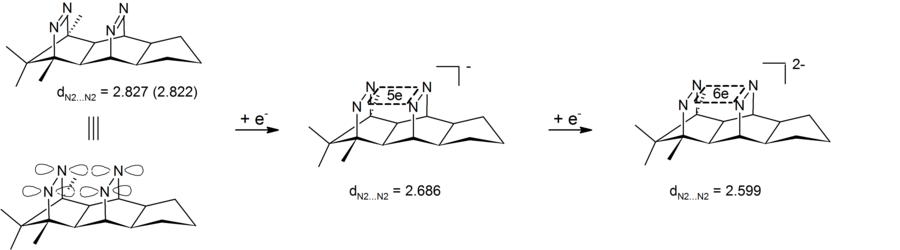
- Fig .: Two-stage reduction of a bisdiazene to a 4-center 6-electron dianion. Distances in Angstrom (X-ray structure for the neutral compound, B3LYP / 6-31G * calculation for the mono- and dianion)
With suitable, highly rigid substrates, amazingly persistent dianions can be generated by 2-electron reduction, in which six electrons are delocalized cyclically in the plane (4-center-6-electron dianions, 4N / 6e). Oxidation of the bisdiazenes to the corresponding bisdiazenetroxides finally yields substrates which are surprisingly easy to oxidize to again unusually persistent dications. In this case it remains to be clarified whether the dications are to be described as further evidence for 2-electron σ-bishomoaromatics (4N / 2e) or as an example of a cubic delocalization in a 4O4N / 10e system.
Individual evidence
- ↑ GKS Prakash, VV Krishnamurthy, R. Herges, R. Bau, H. Yuan, GA Olah, W.-D. Fessner, H. Prinzbach J. Am. Chem. Soc. 1986, 108, 836.
- ↑ GKS Prakash, VV Krishnamurthy, R. Herges, R. Bau, H. Yuan, GA Olah, W.-D. Fessner, H. Prinzbach J. Am. Chem. Soc. 1988, 110, 7764.
- ↑ R. Herges, P. v. R. Schleyer, M. Schindler, W.-D. Fessner J. Am. Chem. Soc. 1991, 113, 3649.
- ↑ P. v. R. Schleyer, H. Jiao: What is Aromtaticity? In: Pure & Applied Chemistry, Vol. 68, 209-218, 1996.
- ↑ K. Exner and P. v. R. Schleyer: Theoretical Bond Energies: A critical Evaluation. In: J. Phys. Chem. A 2001, 105, 3407-3416 and literature cited.
- ^ RV Williams, WD Edwards, P. Zhang, D. Berg, and RH Mitchell: Experimental Verification of the Homoaromaticity of 1,3,5-Cycloheptatriene and Evaluation of the Aromaticity of Tropone and the Tropylium Cation by Use of the Dimethyldihydropyrene Probe. In: Journal of the American Chemical Society 2012 134 (40), 16742-16752.
- ↑ H. Prinzbach, G. Gescheidt, H.-D. Martin, R. Herges, J. Heinze, GK Surya Prakash, GA Olah. "Cyclic electron delocalization in hydrocarbon cages (pagodanes, isopagodanes, (bisseco- / seco -) - (dodecahedradienes))". In: Pure & Applied Chemistry, Vol. 67, No. 5, pp. 673-682, 1995.
- ↑ K. Exner, Highly Closely Ordered Bisdiazenes and Their Oxides. Synthesis, photochemistry, (radical) cations, (radical) anions, templates for aza cages. Dissertation, University of Freiburg i. Br., 1998 ( PDF ).
- ↑ K. Exner, D. Hunkler, G. Gescheidt, H. Prinzbach: Do Nonclassical, Cyclically Delocalized 4N / 5e Radical Anions and 4N / 6e Dianions Exist? - One- and Two-Electron Reduction of Proximate, Synperiplanar Bis-Diazenes. In: Angew. Chem. Indt. Ed. 1998, 37, 1910-1913.
- ↑ K. Exner, O. Cullmann, M. Vögtle, H. Prinzbach, B. Grossmann, J. Heinze, L. Liesum, R. Bachmann, A. Schweiger, G. Gescheidt. In: Cyclic In-Plane Electron Delocalization (σ-Bishomoaromaticity) in 4N / 5e Radical Anions and 4N / 6e Dianions - Generation, Structures, Properties, Ion-Pairing, and Calculations. J. Am. Chem. Soc. 2000, 122, 10650-10660.
- ↑ K. Exner, H. Prinzbach, G. Gescheidt, B. Grossmann, J. Heinze. In: Nonclassical, Cyclically Delocalized 4N / 3e Radical Cations and 4N / 2e Dications: One- and Two-Electron Oxidation of Proximate, syn-Periplanar Bisdiazene Oxides. J. Am. Chem. Soc. 1999, 121, 1964-1965.


