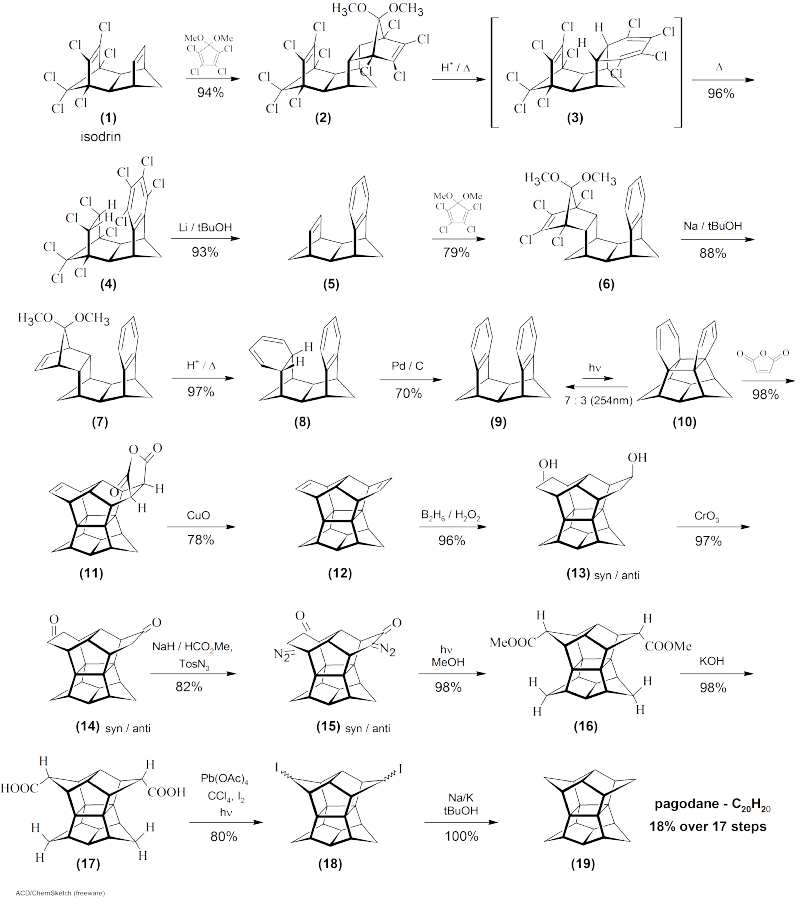
Pagodan
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Pagodan | ||||||||||||
| other names |
Undecacyclo [9.9.0.0 1.5 .0 2.12 .0 2.18 .0 3.7 .0 6.10 . 0 8.12 .0 11.15 .0 13.17 .0 16.20 ] icosan ( IUPAC ) |
||||||||||||
| Molecular formula | C 20 H 20 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 260.38 g mol −1 | ||||||||||||
| Melting point |
243 ° C |
||||||||||||
| solubility |
bad in most organic solvents
|
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Pagodan is a hydrocarbon with the molecular formula C 20 H 20 , the carbon structure of which is reminiscent of a pagoda , from which the name is derived. It is a polycyclic compound with point group D 2 h . The substance is a highly crystalline compound that melts at 243 ° C and is hardly soluble in most organic solvents and only moderately soluble in benzene and chloroform . It can be sublimated at low pressure.
Synthesis and structure
The compound was first synthesized in 1987 by Horst Prinzbach and his colleagues in a 14-step synthesis starting from isodrine 1 . In the course of the synthesis they also isolated [2.2.1.1] pagodane (C 22 H 24 ) 12 and various derivatives.
The entire synthesis can be summarized as follows:
The synthesis shown here can be summarized in 14 one-pot reactions, which offer a total yield of 24%. However, this requires the use of tetrachlorothiophene dioxide instead of tetrachlorodimethoxycyclopentadiene in two early steps. While fewer steps and a higher yield appear attractive at first glance, this approach has been abandoned due to the high costs and the limited availability of the dioxide.
Derivatives
Prinzbach noted that the short name Pagodan is easier to understand than the Baeyer / IUPAC and Chemical Abstracts nomenclature, Undecacyclo [9.9.0.0 1.5 .0 2.12 .0 2.18 .0 3.7 .0 6.10 .0 8.12 .0 11.15 .0 13.17 .0 16.20 ] icosan. As a result, the name Pagodan will continue to be used for a class of compounds whose framework is composed of the same cage of the central 16 carbon atoms. Each of these compounds can be thought of as the result of connecting the eight atoms on each side of the cage by means of four alkyl chains in pairs. The basic designation of the resulting compounds is [ m . n . p . q ] Pagodanes, where m , n , p and q correspond to the number of carbon atoms in these chains. The general formula is C 16+ s H 12 + 2 s , where s is the sum of m , n , p and q . Accordingly, the name of the standard compound C 20 H 20 , which is bridged by four methylene groups ( m = n = p = q = 1), is [1.1.1.1] Pagodan.
Several propellane-like fragments can also be found in the carbon skeleton .
Several derivatives of Pagodan have been synthesized, for example the diketone C 20 H 16 O 2 (melting point above 322 ° C).
Both [1.1.1.1] Pagodan and [2.2.1.1] Pagodan form divalent cations in SbF 5 / SO 2 ClF . In these cations, the electron deficiency is localized on the central cyclobutane ring . These cations were also the first examples in which the phenomenon of σ-bishomoaromaticity could be demonstrated, which was extensively investigated by Horst Prinzbach's group .
Pagodane is an isomer of dodecahedrane and can be chemically converted into it.
Individual evidence
- ↑ a b c d e f g h i j Wolf Dieter Fessner, Gottfried Sedelmeier, Paul R. Spurr, Grety Rihs, H. Prinzbach: "Pagodane": the efficient synthesis of a novel, versatile molecular framework . In: Journal of the American Chemical Society . tape 109 , no. July 15 , 1987, ISSN 0002-7863 , pp. 4626-4642 , doi : 10.1021 / ja00249a029 ( acs.org ).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Elegant Solutions: Ten Beautiful Experiments in Chemistry . Royal Society of Chemistry, Cambridge 2007, ISBN 978-0-85404-674-4 , doi : 10.1039 / 9781847552600 ( rsc.org [accessed August 19, 2020]).
- ↑ Horst Prinzbach, Fabian Wahl, Andreas Weiler, Peter Landenberger, Jürgen Wörth: C20 Carbon Clusters: Fullerene – Boat – Sheet Generation, Mass Selection, Photoelectron Characterization . In: Chemistry - A European Journal . tape 12 , no. 24 , August 16, 2006, ISSN 0947-6539 , p. 6268-6280 , doi : 10.1002 / chem.200501611 ( wiley.com ).
- ^ GKS Prakash: Investigations on intriguing long lived carbocations . In: Pure and Applied Chemistry . tape 70 , no. 10 , October 30, 1998, ISSN 1365-3075 , pp. 2001-2006 , doi : 10.1351 / pac199870102001 ( degruyter.com ).
- ^ GK Prakash, VV Krishnamurthy, Rainer. Herges, Robert. Construction, Hanna. Yuan: Stable carbocations. Part 267. Pagodane dication, a unique 2.pi.-aromatic cyclobutanoid system . In: Journal of the American Chemical Society . tape 108 , no. 4 , February 1986, ISSN 0002-7863 , pp. 836-838 , doi : 10.1021 / ja00264a046 ( acs.org ).
- ^ GK Surya. Prakash, VV Krishnamurthy, Rainer. Herges, Robert. Construction, Hanna. Yuan: Stable carbocations. 273. [1.1.1.1] - and [2.2.1.1] Pagodane dications: frozen two-electron Woodward-Hoffmann transition-state models . In: Journal of the American Chemical Society . tape 110 , no. November 23 , 1988, ISSN 0002-7863 , pp. 7764-7772 , doi : 10.1021 / ja00231a029 ( acs.org ).
- ↑ Wolf-Dieter Fessner, Bulusu ARC Murty, Horst Prinzbach: The Pagodane Route to Dodecahedranes — Thermal, Reductive, and Oxidative Transformations of Pagodanes . In: Angewandte Chemie International Edition in English . tape 26 , no. 5 , May 1987, ISSN 0570-0833 , pp. 451-452 , doi : 10.1002 / anie.198704511 ( wiley.com ).
- ↑ Wolf-Dieter Fessner, Bulusu ARC Murty, Jürgen Wörth, Dieter Hunkler, Hans Fritz: Dodecahedranes from [1.1.1.1] Pagodanes . In: Angewandte Chemie International Edition in English . tape 26 , no. 5 , May 1987, ISSN 0570-0833 , pp. 452-454 , doi : 10.1002 / anie.198704521 ( wiley.com ).