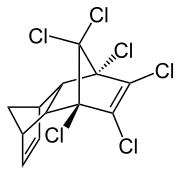
Isodrine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Isodrine | |||||||||||||||
| other names |
1,2,3,4,10,10-hexachloro-1,4,4a, 5,8,8a-hexahydro-1,4- endo -5,8 endo -dimethano-naphthalene |
|||||||||||||||
| Molecular formula | C 12 H 8 Cl 6 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 364.91 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
240-242 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Isodrine is a chemical compound and isomer of aldrin .
Isodrine is produced by a Diels-Alder reaction of 1,2,3,4,7,7-hexachloronorbornadiene with cyclopentadiene . Endrin is formed from isodrine by epoxidation . This reaction can also take place in living things.
Admission
Isodrin is not approved as a crop protection agent in the European Union or Switzerland .
Web links
Individual evidence
- ↑ a b c Entry on isodrine in the GESTIS substance database of the IFA , accessed on November 24, 2014(JavaScript required) .
- ↑ Entry on (1α, 4α, 4aβ, 5β, 8β8aβ) -1,2,3,4,10,10-hexachloro-1,4,4a, 5,8,8a-hexahydro-1,4: 5.8 -dimethanonaphthalene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ External identifiers or database links to 1,2,3,4,7,7-hexachlorbicyclo [2.2.1] hepta-2,5-diene : CAS number: 3389-71-7, EC number: 222-220-8, ECHA InfoCard: 100.020.201 , PubChem : 18820 , ChemSpider : 17771 , Wikidata : Q82857165 .
- ↑ CW Bird, RC Cookson, E. Crundwell: 946. Cyclizations and rearrangements in the isodrin-aldrin series . In: Journal of the Chemical Society (Resumed) . 1961, p. 4809. doi : 10.1039 / JR9610004809 .
- ^ Müfit Bahadir, Harun Parlar, Michael Spiteller: Springer Umweltlexikon . Springer, 2000, ISBN 3-642-56998-6 , pp. 612 ( limited preview in Google Book search).
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national directory of plant protection products in Switzerland ; Retrieved June 25, 2016.

