Boiling analysis
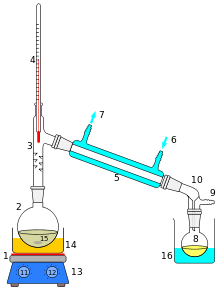
1 : Heating source
2 : Distillation flask
3 : Distillation attachment
4 : Thermometer
5 : Liebig condenser
6 : Cooling water inlet
7 : Cooling water outlet
8 : Round-bottomed flask for the distillate
9 : Pressure equalization
10 : Advance
11 : bath temperature controller
12 : knob speed magnetic stirrer
13 : magnetic stirrer, heating plate
14 : heating bath (water bath, oil bath)
16 : magnetic stirrer, boiling chips
16 : cooling bath, possibly ice bath.

The boiling analysis according to EN ISO 3405 or ASTM D86 determines the boiling behavior of multi-substance mixtures containing hydrocarbons.
execution
The boiling point analysis is a simple direct current distillation in which 100 ml of the sample substance is evaporated in a defined apparatus under controlled heating. The current boiling temperature is recorded at the top of the apparatus (see picture), the vapors are cooled using a cooler, and the volume of condensate collected is determined using a measuring cylinder (not shown correctly in the picture). This creates a boiling temperature / condensate volume relationship, also known as the boiling curve.
This simple, albeit not very precise, method is one of the most important methods for characterizing various mineral oil products and components. The method results in a large number of individual qualities, all of which emerge from the boiling curve:
Start of boiling
The tip of the thermometer is monitored with the aid of a photocell. As soon as the first drop condenses here, the temperature is recorded as the start of boiling (SB, Initial Boiling Point, IBP).
It should be noted that low-boiling components (butane) do not condense, so they only have a slight effect on the start of boiling. Since such substances also uncondensed leave the apparatus, they contribute to the so-called losses ( Losses ) on (s. U.).
Condensate volume
The volume of condensate collected at a certain temperature is an important quality for many mineral oil products. For example, the E70 (see below) is the volume of condensate which was collected at 70 ° C. It is Z. B. for motor gasoline an important quality parameter (with regard to the cold start properties, but also with regard to the limitation of the formation of vapor bubbles, see Vapor Lock Index ).
In general, these condensate volumes are represented by the designation E x , where the abbreviation E comes from the English word evaporated , x is the corresponding temperature (in ° C). Important E-indicators are for example:
- E70, E100, E150 (petrol)
- E205 ( Jet A1 )
- E250, E350 (diesel fuel, heating oil -EL)
Boiling temperature / boiling point
(Not to be confused with the boiling point of pure substances) The temperature at which a certain volume of condensate is collected is called the boiling point. This temperature is represented by the designation Ty, where y is the condensate volume (in%) at the corresponding temperature. Important boiling points include a .:
- T5 (for process control of distillation plants)
- T10, T50, T90 (for the cetane index )
- T95 (for diesel fuel , but also for process control of distillation plants)
End of boiling
The temperature at which no further evaporation takes place is called the end of boiling (SE, English: Final Boiling Point, FBP ). The SE is z. B. specified for petrol and Jet A1.
Residue
Remain a few milliliters after the analysis in the distillation flask back, they will be (engl. As a residue Residue ), respectively. This is z. B. specified for Jet A1.
losses
In most cases, the volume of condensate collected is less than 100 ml, so some parts have not condensed or evaporated (residue). The volume 100 - [Residue] - [trapped condensate volume] is a loss (ger .: Loss refers). Losses are e.g. B. specified for Jet A1.
meaning
An important area of application for boiling analysis is the control of process plants (distillation).
The characterization of end products also plays an important role. A uniform boiling curve without a boiling gap is of great qualitative importance for most fuels. Too many low-boiling components can lead to losses (emission of volatile organic compounds ) and vapor bubbles in the fuel at higher outside temperatures (see volatility index ). High-boiling components can cause engine damage through condensation in the cylinder chamber (thinning of the engine oil).
Others
This determination of the boiling curve is often confused with (much more complicated) methods (e.g. True Boiling Point Distillation , SimDest ). The corresponding (DIN or ASTM) method should always be specified.
Depending on the composition of the material to be examined and the end point of boiling, other standard distillation methods, such as UOP1-87, can also be used in addition to the methods mentioned.
The boiling curve of fuels can also be determined using gas chromatographic methods such as ASTM Method D3710-95.
Individual evidence
- ↑ ASTM .
- ↑ Description of UOP1 - 87 .
- ↑ Overview at Agilent (PDF; 60 kB).
