Sulfoxidation
As sulfoxidation an industrial process for producing refers alkanesulfonates . In the course of the reaction there is an exchange ( substitution ) of hydrogen by -SO 3 H in alkanes . This reaction is a radical substitution .
history
Sulphoxidation was discovered in 1940 in the Hoechst plant of what was then IG Farben AG when paraffins were treated with sulfur dioxide and oxygen when exposed to UV light . This process was further developed between 1941 and 1944, with alkanesulfonates as washing-active substances being produced in small technical plants using the light-water process in Hoechst and the acetic anhydride process in Leuna . It was only ten years after the Second World War that people were interested in sulfoxidation again. The ESSO company developed a new process with the help of gamma rays . In addition, processes with ozone and the addition of small amounts of chlorine as a radical generator were investigated. However, the light-water method then prevailed.
Reaction sequence
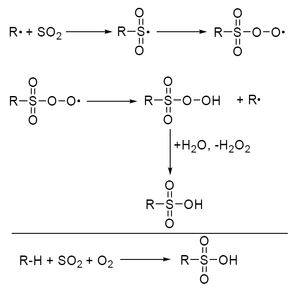
Alkanes react with sulfur dioxide and oxygen under the influence of light or radical starters to form a mixture of alkane sulfonates .
The alkanes are irradiated with UV light at 30 to 38 ° C. in the presence of a mixture of SO 2 / O 2 . Added water hydrolyzes the intermediate alkane persulfonates to sulfonic acids. To avoid higher sulfonated compounds, the conversion is limited to a few percent.
The end products of this radical chain reaction, which takes place industrially on long-chain alkanes, are valuable intermediate products for the production of surfactants.
literature
- H. Ramloch, G. Täuber: Modern Processes of Großchemie: Die Sulfoxidation, Chemie in our time , 13th year 1979, No. 5, pp. 157-163, ISSN 0009-2851
- Falbe (Ed.), “Surfactants in consumer products”, pp. 70-71, Berlin: Springer 1986.