Trialkylboranes
| Trialkylboranes |
|---|
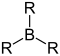 General formula (R = alkyl) |
 Trimethylborane |
 Triethylborane |
Trialkylboranes (BR 3 ) are mostly unstable, air-sensitive compounds. They are therefore often processed further immediately after production.
Manufacturing
There are several methods for the synthesis of trialkylboranes:
- from hydrogen boride (BH 3 ) and alkenes ,
- from boron trihalides - e.g. B. Boron trifluoride (BF 3 ) - with Grignard compounds,
- from trialkyl derivatives of boroxine and
- from trialkylaluminum compounds.
The most atom-efficient method is the addition of hydrogen boride to the C = C double bond of alkenes. So BH 3 reacts z. B. with ethene to triethylborane :
properties
In contrast to diborane (B 2 H 6 ), the trialkylboranes are in monomeric form because dimerization via two-electron three-center bonds is prevented for steric reasons.
use
The oxidation of the trialkylboranes with hydrogen peroxide (H 2 O 2 ) leads to alcohols. So z. B. Triethylborane oxidized to triethylborate and then hydrolyzed to ethanol under the action of sodium hydroxide solution :
Individual evidence
- ↑ a b c Joachim Buddrus, Bernd Schmidt: Fundamentals of Organic Chemistry , 5th edition, de Gruyter Verlag, Berlin, 2015, pp. 176–178, ISBN 978-3-11-030559-3 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 237, ISBN 3-342-00280-8 .
- ^ A b Siegfried Hauptmann: Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 548, ISBN 3-342-00280-8 .
- ↑ a b Otto Albrecht Neumüller (Editor): Römpp Chemie Lexikon , 8th edition, Frank'sche publishing firm, Stuttgart 1983, ISBN 3-440-04513-7 , S. 497th

