Gelding rearrangement
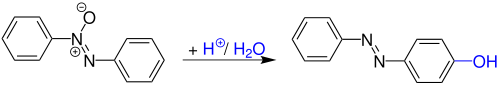
The Wallach rearrangement , also called Wallach transformation , is a name reaction of organic chemistry and was discovered in 1880 by Otto Wallach and L. Belli . This is an acid-catalytic rearrangement of azoxybenzenes to hydroxyazobenzenes .
Overview reaction
Otto Wallach and L. Belli discovered the reaction when they dissolved azobenzene in concentrated sulfuric acid by gently warming it up . After dilution with water, garnet-red, metallic shimmering pyramids fell out.
The benzidine rearrangement is very similar to the Wallach rearrangement. However, strong acids are used for the Wallach rearrangement , whereas weak acids are sufficient for the benzidine rearrangement . Due to the higher concentration of hydrogen ions in strong acids, both nitrogen atoms can be protonated . Kinetic studies have shown that after monoprotonation, the rearrangement rate increases; this indicates a dicationic intermediate. Further kinetic studies showed that a different intermediate product is formed depending on the concentration of the acid. A quinoline intermediate is formed with up to 75% sulfuric acid . From 80% sulfuric acid, a dicationic intermediate is produced. If the concentration is between 75% and 80%, both intermediates are possible.
mechanism
One possible mechanism was formulated by Zerong Wang. The route via dicationic intermediates is shown:
The Wallach rearrangement begins with two hydrogen ions being attached to the azoxybenzene (1) . The first is attached to the negatively charged oxygen atom of the educt. Another is attached to the nitrogen atom without bound oxygen. By dehydration , the dication forms 2 with two mesomeric limiting structures. A phenyl ring is dearomatized, creating a 1,4- quinoid system. In the next step, water accumulates with formation of the oxonium ion 3 . Since this is unstable, a proton splits off. By cleavage of another proton of the second six-Sing is again flavored and there is the desired end product, the hydroxyazobenzene (4) .
application
This reaction is primarily used in the manufacture of hydroxyazobenzene and hydroxyazonaphthalene. These substances are used to color soaps, varnishes, greases and resins.
Individual evidence
- ^ A b Zerong Wang: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 2942-2945 .
- ^ M. Windholz: The Merck Index . Merck & Co., Rakway 1976, ISBN 0-911910-26-3 , pp. ONR-92 .
- ^ O. Wallach, L. Belli: On the conversion of azoxybenzene into oxazobenzene . In: Chem. Ber. tape 13 , 1880, p. 525-527 , doi : 10.1002 / cber.188001301153 .