Weermann dismantling
The Weermann breakdown , more rarely also called the Weermann reaction , is a name reaction in organic chemistry . Rudolf Adrian Weermann reported this reaction for the first time in 1910 . Essentially, these are the reduction of amides to aldehydes with a carbon - atom less. Α-Hydroxy-substituted carboxamides or α, β-unsaturated carboxamides are used as starting material.
Degradation of α-hydroxy-substituted carboxamides
On the one hand, the Weermann degradation can be carried out in the case of α-hydroxy-substituted carboxamides . This includes, for example, sugar .
Overview reaction
When α-hydroxy-substituted carboxamides are broken down, the carbon chain is shortened by one carbon atom and an aldehyde is formed.
The carboxamides react with sodium hypochlorite via hydrolysis to sodium cyanate and an aldehyde. Since the reaction takes place very slowly at room temperature , the reaction mixture is heated to 60-65 ° C. As a result, the sodium hypochlorite is completely consumed within half an hour.
mechanism
The Weermann degradation is probably very similar to the Hofmann rearrangement , as Zerong Wang shows in his book Comprehensive Organic Name Reactions and Reagents .
At the beginning, the carboxamide 1 reacts with the sodium hypochlorite. After water and chloride ions have been split off, an amide ( nitrene ) is formed with two free electron pairs on the nitrogen atom 2 . The intermediate product 3 is then formed by rearrangement . From now on the hydroxy group plays a role. First, hydrolysis takes place. A water molecule attaches to the carbon atom with the number 1 . A hydroxyl group is thereby formed. An acid amide is split off through rearrangement and the desired aldehyde 4 is formed .
Degradation of α, β-unsaturated carboxamides
On the other hand, the Weermann degradation also works with α, β-unsaturated carboxamides, such as acrylamide .
Overview reaction
In the case of the α, β-unsaturated carboxamides, too, the aldehyde is shortened by one carbon atom.
In this reaction, methanol must also be added to the reaction mixture. Thus, instead of hydrolysis, protolysis takes place. Here arise ethenyl - urethanes . This reaction is also allowed to proceed at 60-65 ° C. in order to increase the reaction rate.
mechanism
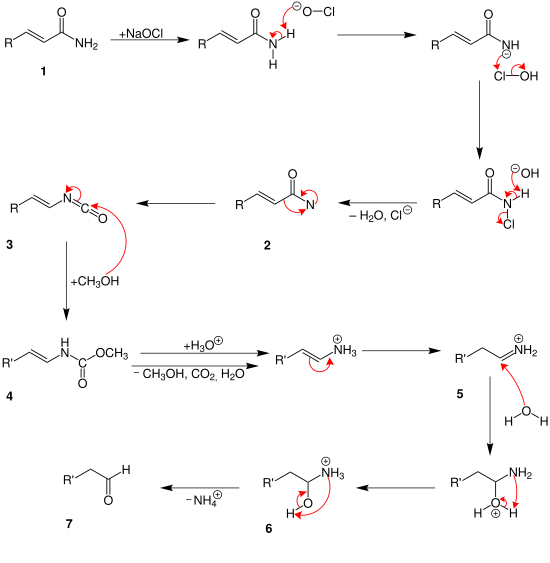
The Weermann breakdown is probably very similar to the Hofmann breakdown, as Zerong Wang shows in his book Comprehensive Organic Name Reactions and Reagents .
At the beginning, the carboxamide 1 reacts with the sodium hypochlorite. After the water and chloride ions have been split off, an amide with two free electron pairs is formed on the nitrogen atom 2 . The intermediate product 3 is then formed by rearrangement . From now on, the α, β-unsaturated bond and the methanol play a role. From here on, the mechanism can run differently. Depending on how many methanol molecules attack the unsaturated bonds. In the mechanism above, the methanol attacks both unsaturated bonds. And so intermediate product 4 is formed . Then carbon dioxide , water, ammonium ions and methanol split off over several steps. In addition, a protolysis takes place. This creates the end product 5 , an aldehyde.
The reaction takes place in exactly the same way up to intermediate 3 . After that, however, only one methanol molecule is deposited on 4 . Protolysis then only splits off water, methanol and carbon dioxide. A hydrocarbon with an ammonium group is formed. Another ammonium ion 5 is created by another rearrangement . This is now hydrolyzed. This creates a hydroxyl group 6 . The end product 7 , an aldehyde, is then formed by splitting off the ammonium ion .
application
The breakdown was mainly used to produce aldehydes that are difficult to synthesize, especially to break down aldonic acid amides into aldoses . As an example, the general breakdown of D -gluconamide to D - arabinose :
In addition, the so-called Weermann test was able to find out whether a hydroxyl group is next to the amido group . However, aldehyde groups are also attacked in this reaction, which is why the yield was always very low. This then increasingly leads to polymerization reactions . In addition, the reverse reaction is often also favored. A relatively good yield is achieved by using urethanes . The Weermann degradation is only of historical chemical importance and is no longer used today due to insufficient yields and large-scale alternative aldehyde syntheses (e.g. hydroformylation ).
Individual evidence
- ^ RA Weermann: Sur une synthèse d'aldéhydes et de l'indol . In: Rec. Trav. Chim. Pays-Bas Belg. tape 29 , 1910, pp. 18-21 , doi : 10.1002 / recl.19100290104 .
- ↑ a b c d e Zerong Wang: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 2946-2950 .
- ^ M. Windholz: The Merck Index . Merck & Co., Rakway 1976, ISBN 0-911910-26-3 , pp. ONR-92 .
- ^ RA Weermann: L'action de l'hypochlorite de sodium sur les amides d'α-hydroxy-acides et de polyhydroxy-acides, ayant un groupe hydroxyle à la place α. Nouvelle method de gradation des sucres . In: Rec. Trav. Chim. Pays-Bas Belg. tape 37 , 1918, pp. 16-22 , doi : 10.1002 / recl.19180370103 .
- ↑ Louis F. Fieser, Mary Fieser: Textbook of organic chemistry . Chemie, Weinheim 1957, p. 409-410 .