Wolffenstein-Böters reaction
The Wolffenstein-Böters reaction is a name reaction in organic chemistry . It is used for the synthesis of dinitrophenol and trinitrophenol (picric acid) and was named after its discoverers, the German chemists Richard Wolffenstein (1864–1929) and Oskar Böters (1848–1912).
Overview reaction
Benzene is reacted with nitric acid in the presence of mercury (II) nitrate . If a 50% nitric acid is used at 30 ° C, then dinitrophenol is formed ; with a 60% nitric acid, trinitrophenol is formed at 100 ° C :
Reaction mechanism
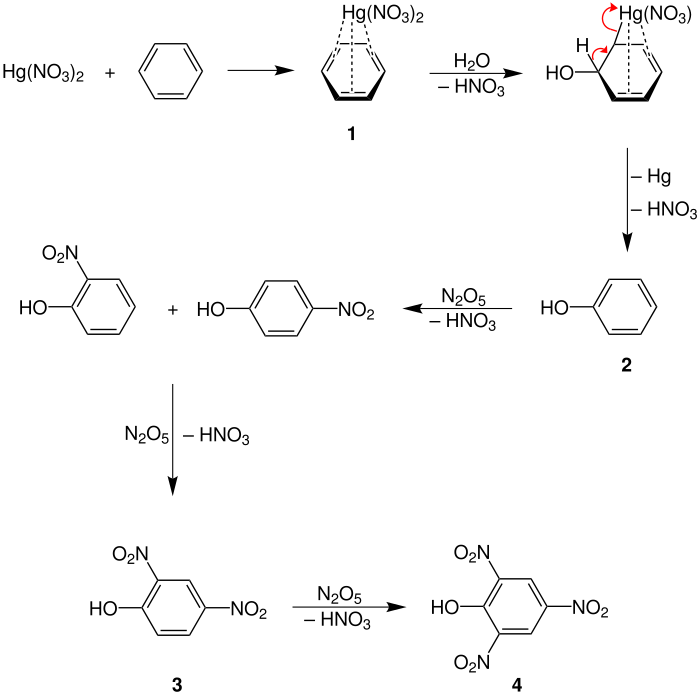
The following reaction mechanism comes from the book "Comprehensive Organic Name Reactions and Reagents" and is only one possible reaction mechanism:
The benzene forms a secondary valence bond with the mercury (II) nitrate , in which the π electrons of the ring system interact with the positively charged mercury ions. The added water adds to the benzene ( 1 ) with elimination of a proton. At the same time, a σ-electron bond is formed between benzene and mercury, with the elimination of a nitrate anion . Through rearomatization , nitric acid is deposited again and phenol is formed ( 2 ).
If ( 2 ) is now reacted with dinitrogen pentoxide , which must first be obtained from nitric acid, nitration takes place in the ortho or para position with elimination of nitric acid. If the nitration is repeated with dinitrogen pentoxide, the other position is nitrated, so that 2,4- dinitrophenol ( 3 ) is formed. Depending on the nitrous oxide concentration and the reaction temperature, it reacts further to form 2,4,6- trinitrophenol ( 4 ).
Mercury problem
This reaction involves working with mercury, which is one of the most toxic substances. In this reaction, the suggested value is 0.42 mol per 100 g of benzene , i.e. 84.24 g. The mercury is recycled with almost no loss.
Individual evidence
- ↑ Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , p. 3083.
- ↑ a b E. E. Aristoff et al: Oxynitration of Benzene to Picric Acid . In: Industrial & Engineering Chemistry . tape 40 , no. 7 July 1948, p. 1281-1290 , doi : 10.1021 / ie50463a024 .
- ↑ Patent DE194883 : Process for the preparation of hydroxylated nitro compounds of the aromatic series. Published on August 4, 1906 , inventor: Richard Wolffenstein, Oskar Böters.
- ↑ Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents, 3 Volume Set , John Wiley & Sons, Hoboken, New Jersey 2009 , p. 3081, ISBN 978-0-471-70450-8 .
- ↑ M. Windholz (Ed.): The Merck Index, Ninth Edition , Merck & Co., 1976 , S. ON-96, ISBN 978-0-911910-26-1 .
- ↑ Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , p. 3082.
- ^ F. Kalberlah, M. Schwarz, P. Jennrich: Mercury: The Underestimated Danger . In: Greenpeace . 2015, p. 5.13 .