Xanthodermine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | Xanthodermine | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 11 H 15 N 4 O 4 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 253.25 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
190-192 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Xanthodermin is a mushroom poison from the carbolic Egerling (synonym carbolic mushroom , Agaricus xanthoderma ) with the very rare structural element of a phenylhydrazine . Structurally related to xanthodermine is the homologous agaritin with a hydroxymethyl instead of the phenolic OH group that occurs in mushrooms .
properties
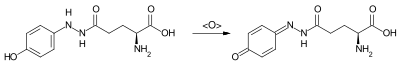
Xanthodermin is autoxidizable. Due to this property, the yellow coloration of the carbolic mushroom is based on damage to the white skin and during preparation. A deep yellow monohydrazone of 1,4-benzoquinone is formed by dehydration (see reaction scheme). The structure was only clarified in 1985 and then synthesized.
Occurrence
Xanthodermin occurs in the widespread Karbol-Egerling (synonym Karbol-Champignon, Agaricus xanthoderma ) in addition to Agaricon and is also responsible for the toxicity and antibiotic effect of the fungus. The building block 4-hydroxyphenylhydrazine and its glucuronide are metabolic products of the likewise toxic and mutagenic phenylhydrazine . Studies of its cytotoxic effects are available for xanthodermin itself. It is much more effective than the ortho isomer found in lichens ( IC 50 = 1.6 μM in B16 cells).
Individual evidence
- ↑ a b c d Angew.Chem. 97, 1063 (1985), Hilbig, Sabine, Andries, Thomas, Steglich, Wolfgang, Anke, Timm; On the chemistry and antibiotic activity of the Carbolegerling (Agaricus xanthoderma) ; doi: 10.1002 / anie.19850971222 ; Chem. Abstr. 1986: 106 184.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Interlaken_1988; Schlunegger, Urs P .; Biologically Active Molecules. Some Chemical Phenomena of Mushrooms and Toadstools (Springer Verlag); ISBN 978-3-642-74582-9 ( online ; from page 4)
- ↑ B. Fischer, J. Lüthy, C. Schlatter: Determination of the content of agaritin in cultivated mushrooms ( Agaricus bisporus ) using high-performance liquid chromatography (HPLC). Journal of Food Study and Research. September 1984, 179 (3), pp. 218-223, doi: 10.1007 / BF01041897 .
- ↑ Bioorg. Med. Chem. Lett. 20: 4582 (2010); Roullier, Catherine; Chollet-Krugler, Marylene; van de Weghe, Pierre; Lohezic-Le Devehat, Francoise; Boustie, J; A novel aryl-hydrazide from the marine lichen Lichina pygmaea: Isolation, synthesis of derivatives, and cytotoxicity assays ; doi: 10.1016 / j.bmcl.2010.06.013 ; Chem. Abstr. 2010: 900 778.