Bohr model: Difference between revisions
Wolfmankurd (talk | contribs) No edit summary |
|||
| Line 8: | Line 8: | ||
==History== |
==History== |
||
In the early [[20th century]], experiments by [[Ernest Rutherford]] and others had established that [[atom]]s consisted of a diffuse cloud of negatively charged [[electron]]s surrounding a small, dense, positively charged nucleus. Given this experimental data, it was quite natural for physicists to consider a planetary model for the atom, the [[Rutherford model]] of |
In the early [[20th century]], experiments by [[Ernest Rutherford]] and others had established that [[atom]]s consisted of a diffuse cloud of negatively charged [[electron]]s surrounding a small, dense, positively charged nucleus. Given this experimental data, it was quite natural for physicists to consider a planetary model for the atom, the [[Rutherford model]] of 1909, with electrons orbiting a sun-like nucleus. However, the planetary model for the atom has several difficulties. For example, the laws of classical (Newtonian) mechanics predict that the electron will release [[electromagnetic radiation]] as it orbits a nucleus. Because the electron would be losing energy, it would be predicted to gradually spiral inwards and collapse into the nucleus. As this occurred, the emission would change in frequency and would be predicted to produce a smear, in frequency, of electromagnetic radiation. However, late [[19th century]] experiments with [[electric discharge]]s through various low-pressure [[gas]]ses in evacuated glass tubes had shown that atoms will emit light (that is, electromagnetic radiation), but only at certain discrete frequencies. |
||
To overcome this and other difficulties in explaining electron motion in an atom, [[Niels Bohr]] proposed, in [[1913]], what is now called the '''Bohr model of the atom'''. Two key ideas were: |
To overcome this and other difficulties in explaining electron motion in an atom, [[Niels Bohr]] proposed, in [[1913]], what is now called the '''Bohr model of the atom'''. Two key ideas were: |
||
Revision as of 06:59, 23 April 2007
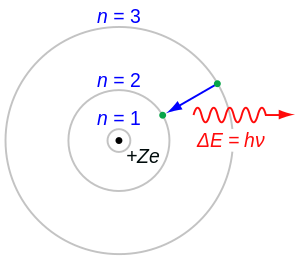
In atomic physics, the Bohr model depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus — similar in structure to the solar system, but with electrostatic forces providing attraction, rather than gravity. This was an improvement on the earlier Plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911). Since the Bohr model is an improvement on the Rutherford model, many sources combine the two referring to the Rutherford-Bohr model.
Introduced by Niels Bohr in 1913, the model's key success was in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen; while the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, but it provided a justification for its empirical results in terms of fundamental physical constants.
The Bohr model is a primitive model of the hydrogen atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics, and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected.
History
In the early 20th century, experiments by Ernest Rutherford and others had established that atoms consisted of a diffuse cloud of negatively charged electrons surrounding a small, dense, positively charged nucleus. Given this experimental data, it was quite natural for physicists to consider a planetary model for the atom, the Rutherford model of 1909, with electrons orbiting a sun-like nucleus. However, the planetary model for the atom has several difficulties. For example, the laws of classical (Newtonian) mechanics predict that the electron will release electromagnetic radiation as it orbits a nucleus. Because the electron would be losing energy, it would be predicted to gradually spiral inwards and collapse into the nucleus. As this occurred, the emission would change in frequency and would be predicted to produce a smear, in frequency, of electromagnetic radiation. However, late 19th century experiments with electric discharges through various low-pressure gasses in evacuated glass tubes had shown that atoms will emit light (that is, electromagnetic radiation), but only at certain discrete frequencies.
To overcome this and other difficulties in explaining electron motion in an atom, Niels Bohr proposed, in 1913, what is now called the Bohr model of the atom. Two key ideas were:
- The electrons travel in orbits and have discrete quantized momenta, and therefore quantized energies. That is, not every orbit is possible but only certain specific ones, at certain specific distances from the nucleus.
- The electrons will not slowly lose energy as they travel, and hence will remain in stable, non-decaying orbits.
The great significance of the model is that it states that the laws of classical mechanics do not apply to the motion of the electron about the nucleus. Bohr proposed that a new kind of mechanics, or quantum mechanics, describes the motion of the electrons around the nucleus. This model of electrons traveling in quantized orbits around the nucleus, however, was replaced with a more accurate model of electron motion about ten years later by the Austrian physicist Erwin Schrödinger and the German physicist Werner Heisenberg.
Other points are:
- When an electron makes a jump from one orbit to another, the energy difference is carried away (or supplied) by a single quantum of light (called a photon) which has an energy equal to the energy difference between the two orbits.
- The allowed orbits depend on quantized (discrete) values of orbital angular momentum, L according to the equation
Where n = 1,2,3,… and is called the principal quantum number, and h is Planck's constant.
Point (2) states that the lowest value of n is 1. This corresponds to a smallest possible radius of 0.0529 nm. This is known as the Bohr radius. Once an electron is in this lowest orbit, it can get no closer to the proton.
Electron energy levels in hydrogen
The Bohr model is accurate only for one-electron systems such as the hydrogen atom or singly-ionized helium. It can also be used for K-line X-ray transition calculations, if other assumptions are introduced (see Moseley's law below). This section uses the Bohr model to derive the energy levels of hydrogen.
The derivation starts with three simple assumptions:
- 1) The energy of an electron in an orbit is the sum of its kinetic and potential energies:
- where , and is the charge of the electron.
- 2) The angular momentum of the electron can only have certain discrete values:
- where n = 1,2,3,… and is called the principal quantum number, h is Planck's constant, and .
- 3) The electron is held in orbit by the coulomb force. That is, the coulomb force is equal to the centripetal force:
To begin, multiply both sides of eq (3) by r to see
The term on the left hand side is the potential energy. So the equation for the energy becomes
Now we just need to figure out what the velocity, v is equal to, so solve eq (2) for r,
Plug this into eq (4),
Then divide both sides by mev to see
Now we can put this value for v into the equation for energy, and then also substitute the values for k and , and we'll obtain the energy of the different levels of hydrogen:
Or, after substituting values for the constants,
Thus, the lowest energy level of hydrogen (n = 1) is about -13.6 eV. The next energy level (n = 2) is -3.4 eV. The third (n = 3) is -1.51 eV, and so on. Note that these energies are less than zero, meaning that the electron is in a bound state with the proton. Positive energy states correspond to the ionized atom where the electron is no longer bound, but is in a scattering state.
Energy in terms of other constants
Starting with what we found above,
We can multiply top and bottom by , and we'll arrive at
or re-grouping them to make it more clear:
From here we can now write the energy level equation in terms of other constants to:
where,
- is the energy level
- is the rest energy of the electron
- is the fine structure constant
- is the principal quantum number.
Rydberg formula
The Rydberg formula, which was known empirically before Bohr's formula, is now in Bohr's theory seen as describing the energies of transitions or quantum jumps between one orbital energy level, and another. Bohr's formula gives the numerical value of the already-known and measured Rydberg's constant, but now in terms of more fundamental constants of nature, including the electron's charge and Planck's constant.
When the electron moves from one energy level to another, a photon is emitted. Using the derived formula for the different 'energy' levels of hydrogen one may determine the 'wavelengths' of light that a hydrogen atom can emit.
The energy of a photon emitted by a hydrogen atom is given by the difference of two hydrogen energy levels:
- where qe is the charge of an electron (1.60 × 10−19 C), nf is the final energy level, and ni is the initial energy level. It is assumed that the final energy level is less than the initial energy level.
Since the energy of a photon is
the wavelength of the photon given off is given by
The above is known as the Rydberg formula. This formula (with all of the numerical constants lumped into a single empirically measured Rydberg constant number R), was known in the nineteenth century to scientists studying spectroscopy, but there was no theoretical justification for the value of R, or for the form of the formula itself, until Bohr derived them, more or less along the lines above. In fact, Bohr's derivation of the empiric Rydberg constant to high accuracy, in terms of other known physical constants, was an early reason for the acceptance of Bohr's model as an advance in understanding.
Moseley's law and calculation of K-alpha X-ray emission lines
Niels Bohr said in 1962, "You see actually the Rutherford work [the nuclear atom] was not taken seriously. We cannot understand today, but it was not taken seriously at all. There was no mention of it any place. The great change came from Moseley."
In 1913 Henry Moseley found an empirical relationship between the strongest X-ray line emitted by atoms under electron bombardment (then known as the K-alpha line), and their atomic number Z. Moseley's empiric formula was found to be derivable from Rydberg and Bohr's formula (Moseley actually mentions only Earnest Rutherford and Antonius Van den Broek in terms of models). The two additional assumptions that [1] this X-ray line came from a transition between energy levels with quantum numbers 1 and 2, and [2], that the atomic number Z when used in the formula for atoms heavier than hydrogen, should be diminished by 1, to (Z-1)2. Moseley wrote to Bohr in puzzlement about this fact, but Bohr at that time was not able to help, thinking that the postulated inner or "K" shell of electrons in elements should have at least four electrons, not two. Thus, Moseley left the matter without an attempt at theoretical explanation. Much later, the effect was realized to be in compensation for the screening effect of the single electron remaining in the lowest atomic energy level, which actually only has two electrons. As noted, Moseley did not choose to speculate on the reason for this even more general screening effect (which was numerically much higher for the L-alpha transition between levels 2 and 3), and the basic mechanism for it which would only become clear later, after further understanding of the correct atomic electron shell structure.
In Bohr's formula for hydrogen above, the charge q4 is a product of the electron charge q2 and the nuclear charge (Zq)2 = q2 Z2. The nuclear charge Z2 may then be factored out as a pure number.
Moseley's law for K-alpha lines is given by the following changes in Bohr's formula:
or
This latter relationship had been empirically derived by Moseley, in a simple plot of the square root of X-ray frequency against atomic number. Moseley's law not only established the objective meaning of atomic number (see Henry Moseley for detail) but, as Bohr noted, it also did more than the Rydberg derivation to establish the validity of the Rutherford/Van den Broek/Bohr nuclear model of the atom, with atomic number as nuclear charge.
The K-alpha line of Moseley's time is now known to be a pair of close lines, written as (Kα1 and Kα2) in Siegbahn notation.
Shortcomings
The Bohr model gives an incorrect value for the ground state orbital angular momentum. The angular momentum in the true ground state is known to be zero. Although mental pictures fail somewhat at these levels of scale, an electron in the lowest modern "orbital" with no orbital momentum, may be thought of as not to rotate "around" the nucleus at all, but mearly to pass back and forth straight "through" it, in a spherical cloud of probability which grows more dense as the nucleus is approached, but which is also characterized by a larger distance where the electron is most likely to be found (due to its slowing as it travels outward, and also volume-shells increase with distance). This radius in hydrogen is equal to the Bohr radius, but since one model is based on the electron having a minimal angular momentum and other on its having none at all, the fact that both return the same distance number, is in some sense a coincidence of nature.
The Bohr model also has difficulty with, or else fails to explain:
- Much of the spectra of larger atoms. At best, it can make predictions about the K-alpha and some L-alpha X-ray emission spectra for larger atoms, if two additional ad hoc assumptions are made (see Moseley's law above). Emission spectra for atoms with a single outer-shell electron (atoms in the lithium group) can also be approximately predicted. Also, if the empiric electron-nuclear screening factors for many atoms are known, many other spectral lines can be deduced from the information, in similar atoms of differing elements, via the Ritz-Rydberg combination principles (see Rydberg formula). All these techniques essentially make use of Bohr's Newtonian energy-potential picture of the atom.
- The relative intensities of spectral lines; although in some simple cases, Bohr's formula or modifications of it, was able to provide reasonable estimates (for example, calculations by Kramers for the Stark effect).
- The existence of fine structure and hyperfine structure in spectral lines, which are known to be due to a variety of relativistic and subtle effects, as well as complications from electron spin.
- The Zeeman effect - changes in spectral lines due to external magnetic fields; these are also due to more complicated quantum principles interacting with electron spin and orbital magnetic fields.
Refinements
Several enhancements to the Bohr model were proposed; most notably the Sommerfeld model or Bohr-Sommerfeld model, which suggested that electrons travel in elliptical orbits around a nucleus instead of the Bohr model's circular orbits. This model supplemented the quantized angular momentum condition of the Bohr model with an additional radial quantization condition, the Sommerfeld-Wilson quantization condition
where p is the generalized momentum conjugate to the angular generalized coordinate q; the integral is the action of action-angle coordinates.
The Bohr-Sommerfeld model proved to be extremely difficult and unwieldy when its mathematical treatment was further fleshed out. In particular, the application of traditional perturbation theory from classical planetary mechanics led to further confusions and difficulties. In the end, the model was abandoned in favour of the full quantum mechanical treatment of the hydrogen atom, in 1925, using Schrödinger's wave mechanics.
However, this is not to say that the Bohr model was without its successes. Calculations based on the Bohr-Sommerfeld model were able to accurately explain a number of more complex atomic spectral effects. For example, up to first-order perturbation, the Bohr model and quantum mechanics make the same predictions for the spectral line splitting in the Stark effect. At higher-order perturbations, however, the Bohr model and quantum mechanics differ, and measurements of the Stark effect under high field strengths helped confirm the correctness of quantum mechanics over the Bohr model.
The Bohr-Sommerfeld quantization condition as first formulated can be viewed as a rough early draft of the more sophisticated condition that the symplectic form of a classical phase space M be integral; that is, that it lies in the image of , where the first map is the homomorphism of Čech cohomology groups induced by the inclusion of the integers in the reals, and the second map is the natural isomorphism between the Čech cohomology and the de Rham cohomology groups. This condition guarantees that the symplectic form arise as the curvature form of a connection of a Hermitian line bundle. This line bundle is then called a prequantization in the theory of geometric quantization.
See also
- Franck-Hertz experiment provided early support for the Bohr model.
- Moseley's law provided early support for the Bohr model. See also Henry Moseley
- Inert pair effect is adequately explained by means of the Bohr model.
- Lyman series
- Schrödinger equation
- Theoretical and experimental justification for the Schrödinger equation
- Balmer's Constant
References
Historical
- Niels Bohr (1913). "On the Constitution of Atoms and Molecules (Part 1 of 3)". Philosophical Magazine. 26: 1–25.
{{cite journal}}: External link in|title= - Niels Bohr (1913). "On the Constitution of Atoms and Molecules, Part II Systems Containing Only a Single Nucleus". Philosophical Magazine. 26: 476–502.
- Niels Bohr (1913). "On the Constitution of Atoms and Molecules, Part III". Philosophical Magazine. 26: 857–875.
- Niels Bohr (1914). "The spectra of helium and hydrogen". Nature. 92: 231–232.
- Niels Bohr (1921). "Atomic Structure". Nature.
{{cite journal}}: External link in|title= - A. Einstein (1917). "Zum Quantensatz von Sommerfeld und Epstein". Verhandlungen der Deutschen Physikalischen Gesellschaft. 19: 82–92. Reprinted in The Collected Papers of Albert Einstein, A. Engel translator, (1997) Princeton University Press, Princeton. 6 p.434. (Provides an elegant reformulation of the Bohr-Sommerfeld quantization conditions, as well as an important insight into the quantization of non-integrable (chaotic) dynamical systems.)
Further reading
- Linus Pauling (1985). General Chemistry, Chapter 3 (3rd ed). Dover Publications. A great explainer of Chemistry describes the Bohr model, appropriate for High School and College students.
- George Gamow (1985). Thirty years that shook Physics, Chapter 2. Dover Publications. A popularizer of physics explains the Bohr model in the context of the development of quantum mechanics, appropriate for High School and College students
- Walter J. Lehmann (1972). Atomic and Molecular Structure: the development of our concepts, chapter 18. John Wiley and Sons. Great explanations, appropriate for High School and College students
- Paul Tipler and Ralph Llewellyn (2002). Modern Physics (4th ed.). W. H. Freeman. ISBN 0-7167-4345-0.





































