Pyrylium

A pyrylium compound is a conjugated 6 membered carbon ring system with one carbon atom replaced by a positively charged oxygen atom forming a salt with a negatively charged counterion [1]. It is the oxygen pendant of benzene and shares with it aromatic properties.
Chemical properties
The carbon to oxygen double bond is also an oxonium ion which due to aromatic stabilization is much less reactive than ordinary oxonium ions. However, the parent compound pyrylium is unstable in neutral water like oxonium salts. Pyrylium cations react with nucleophiles in the 2,4 and 6 positions and may result in ring-opening reactions.
Pyrones
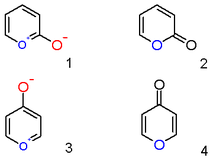
A pyrylium cation with a hydroxyl anion substituent in the 2-position is not the zwitterionic aromatic compound (1) but a neutral unsaturated lactone or a α-pyrone or pyran-2-one (2). Important representatives of this class are the coumarins. Likewise a 4-hydroxyl pyrylium compound is a φ-pyrone or pyran-4-one (4) to which group belongs a compound such like maltol.
Chemical properties
2-Pyrones are known to react with alkynes in a Diels-Alder reaction to form arene compounds with expulsion of carbon dioxide, for example [2]:

Chromenylium ion

The benzo-fused pyrylium ion is also called benzopyrilium or according to IUPAC chromenylium ion. This is the charged version of 1-benzopyran or chromene (IUPAC).
Flavylium ion
In biology the ion 2-phenylchromenylium is referred to as flavylium. A class of flavylium derived compounds are anthocyanins, i.e. pigments which are responsible for the colors of many flowers.
See also
- 6-membered aromatic rings with one carbon replaced by another group: borabenzene,benzene, silabenzene, germanabenzene,stannabenzene, pyridine, phosphorine, pyrylium salt
- pyrans are pyrones lacking the ketone group.
External links
- 2,6-Di-tert-butyl-4-methylpyrylium trifluoromethanesulfonate Organic Syntheses, Coll. Vol. 7, p.144 (1990); Vol. 60, p.34 (1981). Article
References
- ^ Heterocyclic Chemistry T. L. Gilchrist ISBN 0-582-27843-0
- ^ An alkynylboronic ester cycloaddition route to functionalised aromatic boronic esters Patrick M. Delaney, Jane E. Moore and Joseph P. A. Harrity Chem. Commun., 2006, 3323 - 3325, doi:10.1039/b607322k
