Coeliac disease
| Coeliac disease | |
|---|---|
| Specialty | Gastroenterology |
Coeliac disease or celiac disease is an auto-immune disorder of the digestive system that occurs in genetically-predisposed individuals. It is characterised by damage or flattening to all or part of the villi lining the small intestine, which interferes with the absorption of nutrients. This damage is caused by eating anything with gluten (gliadin), a protein found in wheat, rye, and barley (as hordein). A small number of coeliacs (people with coeliac disease) may also react negatively to oats, possibly to the protein in them (avenin). Wheat grain varieties under the names triticale, spelt and kamut also contain gluten.
This condition has several other names, including: cœliac disease (with ligature), c(o)eliac sprue, non-tropical sprue, gluten enteropathy, and gluten intolerance.
Signs and symptoms
The diverse range of coeliac disease symptoms may make it difficult to diagnose. There are over two hundred symptoms that have been identified; not all people have the same symptoms; some people have no symptoms at all; and the symptoms may mimic other diseases. Comprehensive lists are available.[1] [2].
Gastrointestinal or digestive problems occur in some coeliacs. It used to be thought that all coeliacs had diarrhea, weight loss, and nutritional deficiencies, but it is now known that only a small percentage have these symptoms. The wide range of digestive symptoms include everything from canker sores to diarrhea to constipation to nausea. Many of the symptoms may mimic other diseases such as irritable bowel syndrome, reflux, or even Crohn's disease and coeliac may be misdiagnosed as any of these. Other symptoms that may occur are bulky, pale, offensive-smelling stools which may float in the toilet bowl, excess flatulence, infrequent, minor rectal bleeding, or persistent pain in the abdomen.
Some symptoms appear to be caused because the villi are unable to absorb nutrients. Some examples are osteoporosis, damage to teeth enamel, anemia, fatigue, rapid or unexplained weight loss, overweight, failure to thrive or stunted growth in children, etc. Yet other symptoms appear to be emotional, such as depression and irritability. Dermatitis herpetiformis is an itchy blistering skin disease that occurs in some coeliacs and is considered to be an external manifestation of coeliac disease.
While some untreated coeliacs may be symptom-free, they are still doing damage to their small intestines. Regardless of the presence or absence of symptoms, the disorder is associated with an increased risk of osteoporosis, miscarriage, certain types of intestinal cancers, and other auto-immune disorders.
Diagnosis
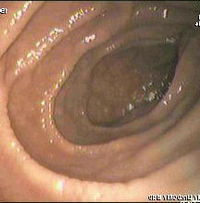
The condition is frequently misdiagnosed or overlooked as it can exhibit multiple symptoms and often the patient or medical staff may not link seemingly unconnected conditions. It is most frequently misdiagnosed when the sufferer complains of diarrhea, persistent indigestion, a rash or irritable bowel syndrome.
There are several tests that can be used to assist in diagnosis. The level of symptoms may determine the order of the tests, but all tests must be done while the person is on a gluten containing diet. Antibodies are reduced and intestinal damage begins to heal immediately upon removing all gluten from the diet, so the risk of misdiagnosis is increased if the person is not eating gluten.
The first tests to be run are usually blood tests. These are discussed in detail below. It is possible for a person to have negative results, however, and still have coeliac disease. If indicated, either because of positive blood tests, or because test results were not consistent by family history or symptoms are, the next step in diagnosis is through a biopsy. This is considered the "gold standard" test that definitively determines if a person has celiac disease.
An upper endoscopy with biopsy of the distal duodenum or jejunum is performed. It is important for the physician to obtain multiple samples from various places throughout the intestine. However, upper endoscopy carries a risk of false negative results. This is because coeliac disease may or may not damage villi throughout the entire small intestine, and upper endoscopy only examines the upper part of the intestine. In a patient whose intestinal damage is located further down, the biopsy may come back negative.
If the endoscopy is positive, then the diagnosis is confirmed. If the endoscopy is negative, the diagnosis is not necessarily excluded.
For people with Dermatitis Herpetiformis (DH) different tests are used, as not all of them will also have damaged villi, though up to 85% do according to the Celiac Disease Foundation. A biopsy of a skin lesion and staining for IgA in the tissues is completed.
Tests
Serology has been proposed as a screening measure, because the presence in the blood of IgA antibodies reactive against gliadin and tissue transglutaminase is indicative of coeliac disease. To show antibodies, the person must be consuming gluten. These tests can be used both to identify coeliac disease and as an annual test to ensure that the celiac is not ingesting gluten.[3]
A thorough workup includes four tests:
- Anti-tissue transglutaminase Antibody (tTG), IgA. This test is sometimes used alone. If this test is positive it is highly likely that the patient has celiac disease. tTG test is not reliable in children before the age of 2.
- Anti-gliadin antibodies (AGA), IgG and IgA. These tests are often useful when testing young symptomatic children, but they are found in fewer coeliacs than Anti-tTG, and their presence does not automatically indicate coeliac disease because they are found in some other disorders. Some people have an IgA deficiency. They are unable to mount an IgA response to any antigen and will have false negative tests for the IgA type celiac tests.
- Anti-endomysial antibodies (EMA), IgA. This test is being replaced by the anti-tTG test because both tests measure the autoantibodies that cause the tissue damage associated with coeliac disease. Many physicians still order this test. This test as tTG test is also not reliable in children before the age of 2.
An older test, the anti-reticulin antibodies (ARA), IgA. IgA Anti-ARA is not ordered as frequently as it once was, because it is less sensitive and less specific than the other tests. It is found in about 60% of people with coeliac disease and 25% of those with dermatitis herpetiformis.
Many doctors do not consider positive blood tests as definitive proof of coeliac disease and require biopsy confirmation. A growing minority consider coeliac disease to be diagnosed where the patient has positive blood tests and shows improved symptoms after the adoption of a gluten-free diet. Because upper endoscopies are expensive and may produce false negative results, this group of doctors considers serology tests and a positive response to eliminating gluten from the diet to be sufficient for diagnosis. The problem with this approach is that patients later commonly want to know if they really have coeliac disease and need to be gluten restricted. A diagnosis with biopsy confirmation at the time of initial diagnosis eliminates this common clinical problem. A small minority of doctors advocate gluten-free diets even for symptom-free patients who have not had an endoscopy but have had a positive blood test, because some confirmed coeliacs are completely symptom-free throughout their lives; in symptom-free patients, the purpose of the diet is to avoid nutritional deficiencies, osteoporosis, and intestinal lymphoma.
Other tests that may assist in the diagnosis are a full blood count, electrolytes, renal function and liver enzymes. Coagulation testing may be useful to identify deficiency of vitamin K, which predisposes patients to hemorrhage.
Pathology
The classic pathology changes of coeliac disease in the small bowel are the following:
- villous atrophy
- mucosal inflammation, often with plasma cells and lymphocytes seen in the lamina propria.
- crypt hyperplasia
The changes classically improve or reverse after gluten is removed from the diet.
Causes
The causes are presently presumed to be:
- Genetic susceptibility to the illness.
- A trigger, which could be one of:
- An environmental agent, probably a virus or other infection
- Stress
- Pregnancy
- Possible exposure to gluten as a young baby before the gut barrier has developed fully. This association is currently under investigation.[4]
Coeliac disease is associated with other autoimmune diseases; these diseases are also probably a combination of susceptibility and infection.
Autoantigens are probably of major importance in the pathogenesis of coeliac disease (transglutaminase), a trait it shares with many other autoimmune diseases; thyroiditis: thyroglobulin, thyroid peroxidase; multiple sclerosis: myelic basic protein, etc. To some extent infectious agents may increase the risk of certain autoimmune diseases (e.g. Coxsackie B in type 1 diabetes). There are few proofs of infections triggering coeliac disease, however. Some researchers have suggested that smoking is protective against coeliac disease. Results on this topic are however inconsistent, and smoking cannot be recommended as a means to avoid developing coeliac disease.
The timing of the first exposure to gluten is also thought to be important. Babies who were introduced to wheat, barley, or rye at any time in the first three months had five times the risk of developing coeliac over those exposed at 4 to 6 months. Those exposed later had a slightly increased risk relative to those exposed at 4-6 months.[4]
Pathophysiology
Antibodies to the enzyme tissue transglutaminase (tTG) are found in an overwhelming majority of cases, and cross-react to gluten[5]. This has led to the theory that they cause the autoimmune like attack on the bowel lining (which is high in tTG), prompted by the continuous stimulation by the α-1-gliaden fragment of gluten (a 33-mer peptide). This reaction happens almost exclusively in patients with human leukocyte antigen types DQ2 and DQ8, which is inherited in families. Over 95% of celiac patients carry one or two of the DQ2 or DQ8 genes. Every person carries two HLA-DQ_ genes, one from their mother and one from their father. About 20% of normal people carry HLA-DQ2, which raises the question of what other factors cause a subgroup of those patients to develop coeliac disease.
The inflammatory process leads to disruption of the structure and function of the small bowel's mucosa, and causes malabsorption (it impairs the body's ability to absorb nutrients and fat-soluble vitamins A, D, E and K from food).
The targets of the immunologic response are gliadin, hordein, and secalin, proteins contained in the gluten component of wheat, barley, and rye respectively. Traditionally, oats have been included in the list as well, but some recent studies have brought into question whether this is necessary. Maize (corn), sorghum, rice and other carbohydrate rich foods, like potatoes and bananas, are safe for a patient to consume. They do not contain gluten and do not trigger the disease.
Treatment
The only treatment is a life-long gluten-free diet. At this time no medication will prevent damage, nor prevent the body from attacking the gut when gluten is present. The disease is controlled by strict adherence to a gluten-free diet, which allows the intestines to heal and resolves all symptoms in the vast majority of cases and, depending on how soon the diet is begun, can also eliminate the heightened risk of osteoporosis and intestinal cancer.
In the vast majority of patients, a strict gluten-free diet will relieve the symptoms. A tiny minority of patients suffer from refractory sprue, which means they do not improve on a gluten-free diet. This may be because the disease has been present for so long that the intestines are no longer able to heal. In other patients, the intestinal damage of coeliac disease may have been aggravated by other problems, such as intolerance to the dietary proteins found in eggs, milk, or soy. Just as a person who is allergic to cats may also happen to be allergic to pollen, a patient with coeliac disease may also happen to have other food intolerances that cause similar symptoms. In rare cases only the complete removal of members of the Gramineae family of plants from the diet will bring about recovery from symptoms.
Clinical trials are underway for a medication that can be taken by coeliacs before eating gluten that will protect against an auto-immune reaction, and hence prevent intestinal damage. Alba Pharmceuticals announced in April, 2006 that Phase I trials for safety were complete. Phase II trials are expected to begin as early at the summer of 2006. The medication is a zonulin inhibitor peptide; people with several autoimmune disorders including type I diabetes, celiac disease, multiple sclerosis and rheumatoid arthritis appear to produce high amounts of zonulin. Zonulin is implicated in causing increased permeability of the intestines as related to coeliac disease.[6]
Epidemiology
Susceptibility to coeliac disease is genetic and many cases are diagnosed in childhood, but the disease can be triggered by environmental factors at any point in life. It is probably most common in the UK, with the average incidence there being 1 in 100 people [1]. With 1 in 250 people diagnosed, Italy also has one of the highest rates of coeliac disease. Those in other countries with British or Italian ancestry are likely to be at risk, owing to the genetic component of the disease. People of African, Japanese, and Chinese descent are rarely diagnosed.
It is estimated that 1 in every 133 to 500 persons (up to 3 million) in the United States and Europe are affected by coeliac disease. One study, by the University of Maryland School of Medicine, found an incidence of 1 out of 133 in a study of 13,000 men and women[7]. The disease is not limited to those of European origin; it is found in other races, but the prevalence is not known. Coeliac disease is more common in women than in men. In symptomatic adults, the average delay between onset of symptoms and diagnosis is estimated at 11 years. This lengthy delay appears to be caused by the variety of symptoms associated with the disease, the fact that some coeliacs have no digestive-tract symptoms at all, and lack of widespread, up-to-date information among doctors.
Epidemiologically, the disease predominates in Northern European populations. Estimates of its frequency among people of European origin range from 1 in 300 to 1 in 500. Some studies indicate that among the Irish, the frequency may be as high as 1 in 133, whilst in the UK the average incidence is 1 in 100. Because it is partly genetic, doctors commonly recommend that the first-degree relatives of diagnosed coeliacs should be tested for the disorder even if they are symptom-free.
In the US, researchers are finding that people with Type I diabetes have a higher risk of coeliac than the other populations. [2] In one study of 125 patients without any specific coeliac symptoms, 12 were confirmed through biopsy as having coeliac.
There is an increased risk of intestinal T-cell lymphoma and osteoporosis in untreated cases. In recent years it has also become more evident that coeliac disease in the pregnant mother could have an adverse effect on the foetus. Offspring to mothers with undiagnosed (and untreated) coeliac disease are more often preterm and low birth weight (weigh less than 2500 grams/5 pounds at birth) than offspring to mothers without coeliac disease. This may be due to the mother's inability to absorb all the nutrients she eats. In children of women with coeliac disease and a gluten-free treatment there seems to be no such risk increase. Women with coeliac disease have fertility similar to that of the general female population, but they often have their babies at an older age.
A number of patients with other diseases are often screened for coeliac disease, including patients with type 1 diabetes, Down's syndrome, Turner's syndrome, irritable bowel syndrome, lupus, and autoimmune thyroid disease.
Social impact
Lifelong diet
The lifelong diet can be difficult and socially troublesome, especially in young patients, but it is crucial in order to avoid serious health consequences. Teenagers in particular occasionally rebel against the dietary strictures and suffer relapses or complications as a result. The widespread use of wheat byproducts in prepared food, soups and sauces can make dining out problematic. This is especially true in the United States, where coeliac disease is less widely-known among the wider population than it is in Europe. However, certain types of restaurant (e.g., Japanese, Thai, Indian, and Latin American) already offer a wide range of gluten-free menu options, and many major restaurant chains have responded to growing awareness of celiac disease by posting information about the gluten content of their menu items on their websites.
It is important for coeliacs to understand that one does not "get over" coeliac disease; it is present for life. As coeliac disease has become better understood, the availability of gluten-free replacements for everyday treats such as muffins, bagels, pasta and the like has continually improved, as has their quality.
It is easy for coeliacs to think they have removed all gluten from their diet, but to continue to consume one or two products that they do not associate with gluten. For example, to eat only gluten-free foods but to continue to drink beer may easily make all that hard work useless. However, even this problem may now be overcome and there are many specialist brews around the world that may be described as gluten free beer.
However, the case of beer raises the main problem of coeliac disease: while the diet is strict and the effects of the disease are serious, the main symptom of the disease can be social isolation with the coeliac afraid to become involved in normal social life. Parties can be difficult, weddings and funerals hard, holidays awkward, a meal out a nightmare, travel is made more stressful, and even the trip to a bar or pub one that requires the individual to be constantly aware of the disease. It is too easy for the coeliac to withdraw from these normal activities, and coeliacs are often working to create normal activities where they can forget the problem. However there are widespread alternatives to beer such as cider and wine, which can be used as an alternative. It is important for newly diagnosed coeliacs to ensure that they do get involved in social activities and are not afraid to "make a fuss".
Religious Issues
Coeliacs and the Eucharist
The Christian sacrament of the Eucharist presents a unique challenge for Christian sufferers of coeliac disease.
In its classical form, the bread and/or communion wafers have traditionally contained wheat flour, and therefore gluten. Coeliacs are therefore presented with a choice between denying themselves a central part of their religious practice or placing themselves at risk of serious illness. In response to this, some makers of communion wafers have begun making gluten-free versions (usually made of rice), which are now widely available. Many churches permit (or have no official policy on) use of these wafers, while other churches do not allow them.
Roman Catholic Position
In particular, Roman Catholic doctrine requires that the Eucharistic host (the communion wafer) must contain at least some unleavened wheat, as did the bread served at the Last Supper. The Catholic Church has approved the use of low-gluten wafers, but even these are not gluten-free. Some Catholic coeliac sufferers have requested permission to use rice wafers; these petitions have so far been denied. [8]
However, official Roman Catholic doctrine is that a Catholic may validly receive communion by consuming a communion wafer and/or the consecrated wine. The Council of Trent decreed that taking either wine or wafer qualifies as a valid Communion:
- For we do not receive in the Sacred Host one part of Christ and in the Chalice the other, as though our reception of the totality depended upon our partaking of both forms; on the contrary, under the appearance of bread alone, as well as under the appearance of wine alone, we receive Christ whole and entire (cf. Council of Trent, Sess. XIII, can. iii).
Therefore, any Catholic can receive the Eucharist in the "fullness of the sacrament" (Catechism, Section 1390) simply in a sip of consecrated wine (even an approved low-alcohol wine); even those who cannot safely consume wheat (or indeed, any other grain) can safely partake of the Eucharist.
The matter has been controversial, however, and on August 22, 1994, the Congregation for the Doctrine of the Faith apparently banned coeliac priests stating, "Given the centrality of the celebration of the Eucharist in the life of the priest, candidates for the priesthood who are affected by celiac disease or suffer from alcoholism or similar conditions may not be admitted to holy orders." After considerable upset and debate, the congregation softened the ruling on July 24, 2003 to "Given the centrality of the celebration of the Eucharist in the life of a priest, one must proceed with great caution before admitting to Holy Orders those candidates unable to ingest gluten or alcohol without serious harm,".
Eastern Orthodox Position
The Orthodox Church also requires that the bread used at the Eucharist be made with wheat flour; here the bread is leavened with yeast. In the Orthodox practice, the consecrated bread and wine are given together from a chalice with a spoon. Some Orthodox coeliac sufferers have been able to receive communion simply by having the priest take only wine in the spoon; others, more sensitive to wheat, have had to have some of the wine set aside before the bread is added to the chalice. This latter case is extremely unusual, and is strictly speaking only permissible with the blessing of the diocesan bishop. While the Orthodox do not have such an explicit rationale as the Roman Catholic Church, their general understanding is that, in the case of exceptions made for the sake of Economy, the Holy Spirit makes up whatever is lacking.
The Church of Jesus Christ of Latter-Day Saints
The Sacrament of the Lord's Supper as observed by members of The Church of Jesus Christ of Latter-Day Saints allows for some flexibility in adapting to the needs of coeliac congregants. Section 27 of the church's cannonical Doctrine and Covenants reads:
- "it mattereth not what ye shall eat or what ye shall drink when ye partake of the sacrament, if it so be that ye do it with an eye single to my glory—remembering unto the Father my body which was laid down for you, and my blood which was shed for the remission of your sins."
As awareness of celiac disease has increased, many congregations have made allowance for Coeliac congregants where necessary by permitting gluten-free breadstuff to be used, sometimes alongside regular breads (where possible).
Coeliacs and Passover
The Jewish festival of Pesach (Passover) may present problems with its obligation to eat matzo. Matzo is normally made from wheat or other gluten-containing grains. Oat matzo is now available, however. Many products prepared for Passover are also non-gebrokts, which means they are free of wheat, barley, spelt, oats, and rye. Potato starch is the primary starch used to replace the grains. These products make it easier to participate in Passover.
Specialist breads, pastas, and beer
Coeliac disease may isolate individuals due to dietary strictures, but manufacturers are increasingly producing a wide range of very acceptable breads, and some gluten-free pastas are virtually indistinguishable from wheat pasta. Restaurants are beginning to offer gluten free menus. Gluten free beer is available and there is now a range of ales and lagers to choose from.
Around the world standards of "gluten free" vary. For example, while in the United Kingdom a beer with less than 20 parts per million gluten (20ppm) is "gluten free", in Australia it is not possible to describe any product as such if any gluten can be detected at all. Similarly, some "gluten free" breads can contain low levels of gluten in one country, in another they would contravene labelling or food standards legislation.
It is important, however, for consumers of all "low gluten" foods and beverages to inform their consultant, and to ensure that even if the obvious symptoms are absent, there are no other negative effects continuing that they are unaware of. While one may feel better, even the smallest amounts of gluten can worsen osteoporosis risk or predispose for malignancy.
While large scale commercial beers are out of the question for coeliacs[9] [10] [11], the development of a range of gluten free beers is "an example of celiacs working together to socialize normally and avoid isolation caused by their special dietary needs. It also represents part of the return to a ‘normal’ life"[12].
See also
References
- The Celiac Disease Foundation
- Celiac.com
- University of Maryland Center for Celiac Research
- Icors Celiac Disease Listserve - formerly St.John's
- The Genetics of Celiac Disease
- Psychological Disorders and Celiac Disease
- Foods and Ingredients That Celiacs Must Avoid
- Q&A on Serological Testing and Celiac Disease
- A Short Introduction to Holy Communion and Celiac Sprue Disease from the United States Conference of Catholic Bishops
- Coeliac Disease information and recipes for people with coeliac disease.
- National Foundation for Celiac Awareness A resource of living with Celiac Disease
- A History of the condition
- National Digestive Diseases Clearinghouse
- Gluten Free Beer Resource
Footnotes
- ^ "Symptoms of Celiac Disease". Celiac Sprue Association/United States of America, Inc. (CSA). January 1 2004. Retrieved 30 May 2006.
{{cite web}}: Check date values in:|date=(help) - ^
"Celiac disease - sprue". MedlinePlus Medical Encyclopedia. National Institutes of Health. October 27 2005. Retrieved 30 May 2006.
{{cite web}}: Check date values in:|date=(help) - ^ "Common Questions and Answers on Serologic Tests". Celiac website on enabling.org. 4 December 2001. Retrieved 30 May 2006.
- ^ a b Norris JM, Barriga K, Hoffenberg EJ, Taki I, Miao D, Haas JE, Emery LM, Sokol RJ, Erlich HA, Eisenbarth GS, Rewers M. (2005). "Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease". JAMA. 293 (19): 2343–2351. PMID 15900004.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 1997;3:797-801. PMID 9212111.
- ^
Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE. (2000). "Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease". The Lancet. 355 (9214): 1518–1519. doi:10.1016/S0140-6736(00)02169-3. PMID 10801176.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Prevention magazine September 2004: Multi-center USA Study
- ^ Associated Press (December 8, 2004). "Girl with digestive disease denied Communion". MSNBC. Microsoft. Retrieved 30 May 2006.
- ^ "Ask the Beer Fox - Is Straub's Beer Gluten Free ?". Carolyn Smagalski, Bella Online. 2006.
{{cite web}}: Text "Carolyn Smagalski" ignored (help) - ^
"Is Nigerian Guinness Gluten Free ?". Carolyn Smagalski, Bella Online. 2006.
{{cite web}}: Text "Carolyn Smagalski" ignored (help) - ^
"Ask the Beer Fox – Is Standard Lager Beer Safe for Coeliacs?". Carolyn Smagalski, www.glutenfreebeerfestival.com. 2006.
{{cite web}}: Text "Carolyn Smagalski" ignored (help) - ^
"First-Ever Gluten-Free Beer Festival Quenches Celiacs' Thirst". Robert La France, Glutenfreeda.com. 2006.
{{cite web}}: Text "Robert La France" ignored (help)
