Arene oxides
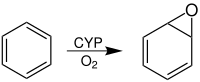
Arene oxides are chemical compounds in which one of the “ double bonds ” of an aromatic ring system has been converted into an epoxide . The simplest arene oxide is benzene oxide .
This conversion takes place under the catalytic action of an enzyme in the body when foreign aromatic substances, such as. B. benzene , get into this. This is done in order to create a water-soluble compound which can ultimately be excreted via the kidneys ( biotransformation ). The enzyme which converts aromatic hydrocarbons into arene oxides and thus has a detoxifying effect is one of the cytochrome P450 enzymes (also CYP). An arene oxide can either behave like an epoxide and form an addition product when attacked by a nucleophile , or it rearranges to a phenol.
Reactions
Benzene ( 1 ) is oxidized in the body (P-450), creating oxirane 2 . Benzene oxide ( 2 ) and oxepine ( 3 ) have a chemical equilibrium in a ratio of about 1: 1. The equilibrium between 2 and 3 is a valence tautomerism equilibrium.
- 2 or 3 reacts to form a hydroquinone ( 6 ) (step C ) and can be further oxidized to a quinone (polyphenol oxidase: reaction mechanism see quinones ).
- The hydrolysis of the epoxide group - by an epoxide hydrolase ( B ) (see benzo [ a ] pyrene ) - produces catechol which can be further oxidized to benzoquinone ( 5 ).
- This rearranges to phenol ( 4 ): arene oxide rearrangement ( A )
Mechanism of the arene oxide rearrangement
The three-membered ring of benzene oxide ( 1 ) accepts a proton from a proton donor . The more stable the carbocation ( 2 ) formed, the easier the ring will open. An enone ( 3 ) is formed by a 1,2-hydride shift . The release of a proton into the solution leads to the formation of phenol ( 4 ) with rearomatization .
Individual evidence
- ↑ a b Paula Yurkanis Bruice: Organic Chemistry , Pearson Education Inc., 2011, 5th edition, p 421, ISBN 978-3-8273-7190-4 .
- ↑ E. Vogel , H. Günther: Benzoloxid-Oxepin-Valenztautomerie In: Angewandte Chemie , Volume 79, 1967, pp. 429-446. doi : 10.1002 / anie.19670791002 .
- ↑ Structure-effect thinking in chemistry - an opportunity for more sustainability, Chapter 5, Industrial Chemicals, p. 317 (PDF; 78 kB) ( Memento of the original from December 19, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. , accessed March 26, 2013.