Arens-van-Dorp reaction
The Arens-van-Dorp reaction is a name reaction of organic chemistry developed by the Dutch chemists JF Arens and DA van Dorp . It describes the synthesis of α, β- unsaturated aldehydes and represented a first successful step towards vitamin A synthesis around 1947. Also in 1947, O. Isler developed a modification, the so-called Isler modification, which enables the industrial synthesis of Vitamin A should make it easier.
Overview reaction
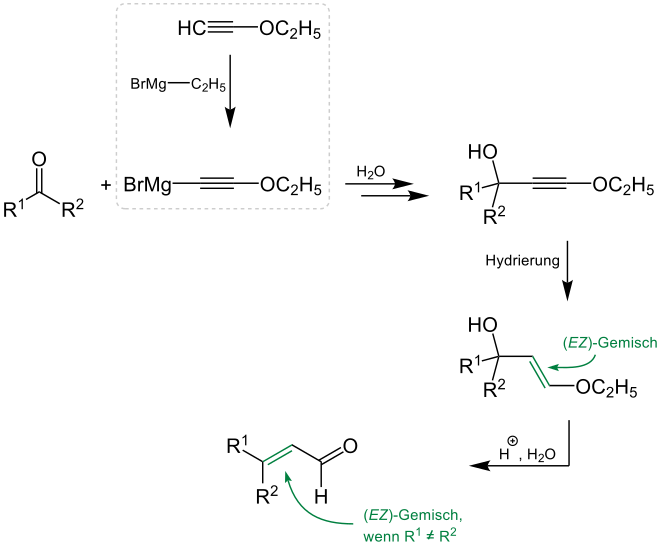
The Arens-van-Dorp reaction describes the reaction of a carbonyl compound and ethoxyacetylene in the form of a Grignard compound to form the α, β- unsaturated aldehyde. The literature essentially lists reactions for the synthesis of vitamin A, in which a ketone is used as the starting material. It is not clear from the literature whether, for example, aldehydes can also function as starting materials. A simplified representation of the Arens-van-Dorp reaction looks like this (where R 1 and R 2 are organic radicals in the following):
Considered in detail, the Arens-van-Dorp reaction proceeds from the carbonyl compound via a Grignard reaction to the alkyne derivative. The production of the Grignard compound can be derived from the work of the chemists TL Jacobs, R. Cramer, T. Weiss and JE Hanson, in which the synthesis of phenoxyacetylene magnesium bromide and other compounds starting from acetylene ethers is described. The chemists MN Shchukina and IA Rubtsov also reported on the synthesis from ethoxyacetylene and ethylmagnesium bromide listed here in 1948. A subsequent catalytic hydrogenation of the alkyne derivative and the reaction with a weak acid leads to the α, β- unsaturated aldehyde:
The Isler modification does not carry out a Grignard reaction and instead uses the reaction of a β- chlorovinyl ether with lithium amide to produce lithium ethoxyacetylene , which is then also mixed with a ketone. The resulting alkyne derivative can then be hydrogenated according to the Arens-van-Dorp reaction and reacted further:
Reaction mechanism
The literature essentially provides information about the course of the Arens-van-Dorp reaction and less about the mechanism. Since the course of the reaction to the α, β- unsaturated carbonyl compound is shown above, only the mechanism of the Isler modification will be discussed below.
Isler modification
With double deprotonation of the β- chlorovinyl ether ( 1 ) by the lithium amide, the chloride is split off and the triple bond is formed. The lithium ethoxyacetylene ( 2 ) formed in this way undergoes a nucleophilic attack on the carbonyl carbon atom of the carbonyl compound. The work-up of the alkyne derivative is not described in detail in the literature. One possibility for working up a lithium alcoholate would be to react it with dilute acid. The alkyne derivative ( 3 ) present after working up can now be further processed by means of catalytic hydrogenation in accordance with the Arens-van-Dorp reaction.
Atomic economy
So that in terms of both the Arens-van Dorp-reaction, as are also formed in the Ilser modification byproducts atom-economical considerations can be spoken of efficient reactions do not. This is due, among other things, to the occurrence of salt waste. In addition, the generation of predominantly organic waste in the context of the Grignard reaction should be considered.
Ethoxyacetylene, as a starting material for the synthesis of the Grignard compound, is also a difficult-to-produce starting material. The lithium ethoxyacetylene used as an alternative in the context of the Isler modification is easier to produce, according to the literature. The formation also works in situ and the reactivity is similar to that of the Grignard compound. The sensitivity of the Grignard reaction to water also gives the Isler modification advantages in industrial processing.
Economic use
The simplified synthesis in the sense of the Isler modification was implemented in the industrial sector by the pharmaceutical company Hoffmann-La Roche . At the end of the 20th century, Hoffmann-La Roche, at that time the world's leading producer of vitamin A, came under fire in the context of investigations by the European Commission because of price agreements with other leading companies. The companies BASF and Rhône-Poulenc also belonged to the cartel . In 2001 fines of several hundred million euros were imposed. Today vitamin A is mainly produced by DSM and BASF.
Individual evidence
- ^ A b c Daniel Zerong Wang: Comprehensive Organic Name Reactions and Reagents . tape 1 . John Wiley & Sons, Inc., Hoboken New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 100-102 .
- ↑ a b c d e f g h J. F. Arens, DA van Dorp: A new method for the synthesis of α, β-unsaturated aldehydes. In: Recueil des travaux chimiques des Pays-Bas. Volume 67, 1948, pp. 973-976.
- ↑ a b c d e f g h Gemma L. Parker, Laura K. Smith, Ian R. Baxendale: Development of the industrial synthesis of vitamin A. In: Tetrahedron. Volume 72, No. 13, 2016, pp. 1645–1652, doi: 10.1016 / j.tet.2016.02.029 .
- ↑ a b O. Isler, W. Huber, A. Ronco, M. Kofler: Synthesis of Vitamin A. In: Helvetica Chimica Acta Volume 30, No. 6, 1947, pp. 1911-1927, doi: 10.1002 / hlca. 19470300666 .
- ^ Thomas L. Jacobs, Richard Cramer, FT Weiss: Acetylenic Ethers. I. Phenoxyacetylenes. In: The Journal of the American Chemical Society. Volume 62, No. 7, 1940, pp. 1849-1854.
- ^ Thomas L. Jacobs, Richard Cramer, John E. Hanson: Acetylenic Ethers. II. Ethoxy- and butoxy-acetylenes. In: The Journal of the American Chemical Society. Volume 64, No. 1, 1942, pp. 223-226.
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren, Peter Wothers: Organic Chemistry. Oxford University Press Inc., New York 2001, ISBN 0-19-850346-6 , pp. 213 .
- ↑ MN Shchukina, IA Rubtsov: Syntheses through Ethoxyacetylenemagnesium Bromide. In: Zhurnal Obshchei Khimii. Volume 18, 1948, pp. 1645-1652.
- ^ Sven Strunk, Manfred Schlosser : Wittig Rearrangement of Lithiated Allyl Aryl Ethers: A Mechanistic Study. In: European Journal of Organic Chemistry. Volume 2006, No. 19, 2006, pp. 4393-4397, doi: 10.1002 / ejoc.200600304 .
- ↑ a b c Bradford P. Mundy, Michael G. Ellerd, Frank G. Favaloro Jr .: Name Reactions and Reagents in Organic Synthesis. Second edition . John Wiley & Sons, Inc., Hoboken New Jersey 2005, ISBN 0-471-22854-0 , pp. 262 .