Büchner-Curtius-Schlotterbeck reaction
The Büchner-Curtius-Schlotterbeck reaction (also Schlotterbeck reaction ) is a name reaction in organic chemistry that was first described in 1885 by Eduard Buchner and Theodor Curtius . A closer look was taken in 1907 by Fritz Schlotterbeck . It is a reaction between a carbonyl compound ( aldehyde or ketone ) and a diazoalkane to form new aldehydes, ketones or epoxides . An extension of the reaction is the synthesis of β- ketoesters as a product of the condensation reaction of aldehydes with diazoesters.
Overview reaction
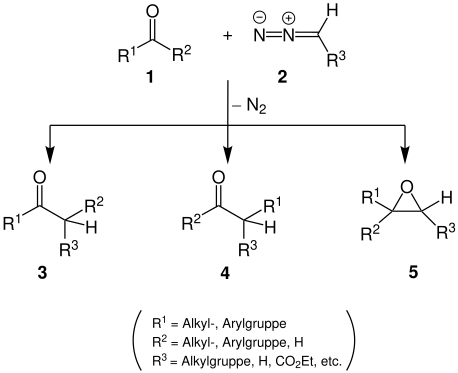
In the Schlotterbeck reaction, an aldehyde or ketone 1 and a diazoalkane 2 react to form an aldehyde or ketone 3 and / or 4 and / or an epoxide 5 . The ratio of the reaction products depends on the reactants used and the reaction conditions.
Reaction mechanism
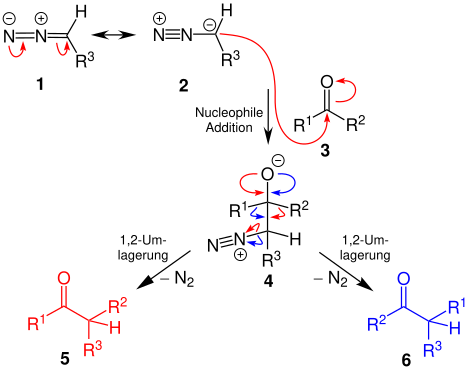
The diazo compound can be represented in resonance structures 1 and 2 . The lone pair of electrons on the carbon atom , which is present next to the nitrogen atom in compound 2 , attacks the carbonyl compound 3 nucleophilically . A tetrahedral intermediate product 4 is formed which decomposes with elimination of gaseous nitrogen and a 1,2 rearrangement to give the carbonyl compounds 5 and / or 6 .
The reaction mechanism for the formation of the epoxide 7 proceeds analogously as a nucleophilic addition up to the formation of the tetrahedral intermediate 4 .
Sample reaction
Via the Schlotterbeck reaction, ethyl 4,4,4-trichloroacetate and ethyl 3- (trichloromethyl) glycidate can be obtained in a ratio of 1: 9 and a yield of 80.4% from chloral and ethyl diazoacetate.
Atomic economy
In the Schlotterbeck reaction essentially only nitrogen gas is produced in stoichiometric amounts as waste material, which means that the atomic efficiency can be classified as relatively good. When considering economic aspects, however, it should be noted that this reaction often does not give clean products, since the formation of various reaction products is possible and can only be influenced to a limited extent.
modification
An extension of the Schlotterbeck reaction enables a direct conversion of aldehydes to β-ketoesters. This is the case, for example, in the reaction of aldehydes with ethyl diazoacetate in the presence of a catalytic amount of tin (II) chloride . The kidney stone reaction , a reaction of diazoalkanes and carboxylic acid halides to form α-halomethyl ketones, can also be viewed as an extension of the Schlotterbeck reaction.
Individual evidence
- ^ E. Buchner, Th. Curtius: Synthesis of ketonic acid ethers from aldehydes and diazoacetic ethers . In: Reports of the German Chemical Society . tape 18 , no. 2 , July 1885, p. 2371-2377 , doi : 10.1002 / cber.188501802118 .
- ^ Fritz Schlotterbeck: Conversion of aldehydes into ketones by diazomethane. (Reply to Mr. H. Meyer) . In: Reports of the German Chemical Society . tape 40 , no. 2 , March 1907, p. 1826-1827 , doi : 10.1002 / cber.19070400285 .
- ↑ a b c d Schlotterbeck Reaction . In: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, Inc., Hoboken, NJ, USA 2010, ISBN 978-0-470-63885-9 , pp. 2491-2494 , doi : 10.1002 / 9780470638859.conrr563 .
- ↑ Büchner-Curtius-Schlotterbeck reaction . In: Name Reactions . Springer Berlin Heidelberg, Berlin, Heidelberg 2006, ISBN 978-3-540-30030-4 , pp. 94-95 , doi : 10.1007 / 3-540-30031-7_46 .
- ↑ David K. Wald, Madeleine M. Joullié: Trichloroacetoacetates. I. Synthesis and Reactions of Ethyl and β, β, β- Trifluoroethyl Trichloroacetoacetates . In: The Journal of Organic Chemistry . tape 31 , no. October 10 , 1966, p. 3369-3374 , doi : 10.1021 / jo01348a060 .
- ↑ Christopher R. Holmquist, Eric J. Roskamp: A selective method for the direct conversion of aldehydes into .beta.-keto esters with ethyl diazoacetate catalyzed by tin (II) chloride . In: The Journal of Organic Chemistry . tape 54 , no. July 14 , 1989, p. 3258-3260 , doi : 10.1021 / jo00275a006 .
- ↑ Douglas Arthur Clibbens, Maximilian kidney stone: CLXV.-The action of diazomethanes on some aromatic acyl chlorides . In: J. Chem. Soc., Trans. Volume 107 , no. 0 , 1915, p. 1491-1494 , doi : 10.1039 / ct9150701491 .