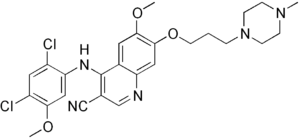
Bosutinib
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Bosutinib | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 26 H 29 Cl 2 N 5 O 3 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 530.45 g mol −1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Bosutinib ( Pfizer ), previously referred to as SKI-606 , is a drug from the class of tyrosine kinase inhibitors .
Bosutinib inhibits various tyrosine kinases in humans , mainly from the Abl and Src families. On September 4, 2012, bosutinib was approved by the American Food and Drug Administration (FDA) for the treatment of patients with chronic myeloid leukemia (CML) in whom other tyrosine kinase inhibitors (e.g. imatinib ) are ineffective or cannot be tolerated . Bosutinib is marketed as a medicinal product in the United States under the name Bosulif . The usual dosage is 500 mg once a day. The approval was primarily based on the results of the BELA study, which had shown comparable efficacy of bosutinib and imatinib. On March 27, 2013, bosutinib was approved by the European Commission for the treatment of patients with CML who have already received at least one tyrosine kinase inhibitor and for whom imatinib, nilotinib and dasatinib are not considered useful by the attending physician. The approval was granted on the condition that the pharmaceutical company had to provide further evidence of the benefits of the drug. In addition, annual evaluations are to take place by the European Medicines Agency .
Bosutinib's property as an Src kinase inhibitor makes it a potential therapeutic agent for the treatment of breast cancer . In breast cancer - as in many other human tumors - the activity of the Src kinases in the tumor tissue is significantly increased. The same applies to colorectal cancer (colon cancer).
Early benefit assessment
In Germany, since 2011, newly approved drugs with new active ingredients must be subjected to an " early benefit assessment " by the Federal Joint Committee (G-BA) in accordance with Section 35a SGB V if the pharmaceutical manufacturer wants to achieve a higher sales price than just the fixed amount . Only if there is an additional benefit can the pharmaceutical manufacturer negotiate a price with the umbrella association of statutory health insurance companies. The dossier evaluations, on the basis of which the G-BA makes its decisions, are created by the Institute for Quality and Efficiency in Health Care (IQWiG) .
After expanding the area of application and revoking orphan drug status, bosutinib underwent two early benefit assessments in 2018. First, it was compared with imatinib or nilotinib or dasatinib in the new therapeutic area (treatment of adults with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in the chronic phase). According to the G-BA decision, an additional benefit compared to this ACT is not proven in the first line.
By exceeding the sales limit for the simplified benefit assessment for orphan drugs, for which an additional benefit was initially assumed to be given in 2013, the original field of application (adults with Ph + CML in the chronic phase, accelerated phase and blast crisis, those with at least one tyrosine kinase inhibitor pretreated and for whom imatinib, nilotinib and dasatinib are not considered to be suitable treatment options) a regular early benefit assessment was carried out. According to the G-BA decision, an added benefit compared to the ACT ponatinib has not been proven for this patient group either.
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Bosutinib
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of 4 - [(2,4-dichloro-5-methoxyphenyl) amino] -6-methoxy-7- [3- (4-methylpiperazin-1-yl) propoxy] quinoline- derived from a self-classification by the distributor is shown. 3-carbonitrile in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 28, 2019.
- ↑ Bosutinib data sheet from Sigma-Aldrich , accessed on February 16, 2020 ( PDF ).
- ↑ FDA approves new orphan drug for chronic myelogenous leukemia. FDA, September 4, 2012, accessed December 25, 2012 .
- ↑ Cortes JE, Kim DW, Kantarjian HM, Brümmendorf TH, Dyagil I, Griskevicius L, Malhotra H, Powell C, Gogat K, Countouriotis AM, Gambacorti-Passerini C: Bosutinib Versus Imatinib in Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia: Results From the BELA Trial. Journal of Clinical Oncology 2012; 30: 3486-3492 PMID 22949154 .
- ↑ Appendix 1: Summary and Characteristics of the Medicinal Product. (PDF; 520 kB) EMA, accessed on May 29, 2013 .
- ^ A. Vultur et al.: SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol. Cancer. Ther. (2008) 7 (5): pp. 1185-1194, PMID 18483306 .
- ↑ AM Coluccia et al .: SKI-606 decreases growth and motility of colorectal cancer cells by preventing pp60 (c-Src) -dependent tyrosine phosphorylation of beta-catenin and its nuclear signaling. Cancer Res. (2006) 66: pp. 2279-2286, PMID 18483306 .
- ↑ A18-33 Bosutinib (chronic myeloid leukemia) - Benefit assessment according to Section 35a SGB V; Accessed March 26, 2020.
- ↑ Benefit assessment procedure for the active ingredient bosutinib (new area of application: chronic myeloid leukemia, Ph +, first line); Accessed March 26, 2020.
- ↑ A18-54 Bosutinib (pretreated chronic myeloid leukemia) - benefit assessment according to § 35a Social Code Book V; Accessed March 26, 2020.
- ↑ Benefit assessment procedure for the active ingredient bosutinib (assessment after revocation of the orphan drug status: chronic myeloid leukemia, Ph +); Accessed March 26, 2020.