Bovichinones
Bovichinones ( English boviquinones ) are chemical compounds that belong to the group of quinones . The compounds are found in various mushrooms and give them a characteristic orange-red color.
Chemical structure
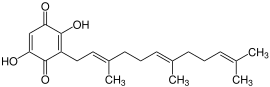
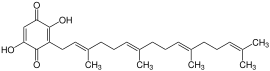
The molecules of the boviquinones are benzoquinone - derivatives . The quinones are substituted with two hydroxyl groups to form a 2,5-dihydroxy-1,4-benzoquinone. In addition, there is a polyprenyl side chain in the 3-position , which is formally derived from isoprene . Depending on the length of the side chain, this results in different bovichinones.
The best-known representatives of the bovichinones are Bovichinon-3 and Bovichinon-4. The molecules of the two substances have the chemical structure already described. While the molecules of bovichinone-3 have a polyprenyl side chain, which consists of three isoprene units, the side chain of bovichinone-4 has a length of 4 linked isoprene units.
Natural occurrence
Bovichinon-3 is found in Chroogomphus rutilus and other Chroogomphus species such as Chroogomphus helveticus .

|
|
|
|
Chroogomphus rutilus , structural formula of bovichinone-3
|
||
Bovichinon-4, on the other hand, is mainly found in the Suillus bovinus .

|
|
|
|
Suillus bovinus , structural formula of bovichinone-4
|
||
As a coloring agent, the chemical compounds ensure the color of the various mushrooms.
Individual evidence
- ↑ a b c Entry on Bovichinone. In: Römpp Online . Georg Thieme Verlag, accessed December 7, 2017.
- ↑ a b Andrea Mühlbauer, Jürgen Beyer, Wolfgang Steglich : The Biosynthesis of the Fungal Meroterpenoids Boviquinone-3 and-4 follows Two Different Pathways . Tetrahedron Letters 39, 1998, doi: 10.1016 / S0040-4039 (98) 01039-9 , pp. 5167-5170.
- ↑ Ramona Heinke, Norbert Arnold, Ludger Wessjohann, Jürgen Schmidt: Negative ion tandem mass spectrometry of prenylated fungal metabolites and their derivatives . In: Analytical and Bioanalytical Chemistry, Springer-Verlag, January 2013, DOI: 10.1007 / s00216-012-6498-1 , pp. 177-189.
