Boyland Sims Oxidation
The Boyland-Sims oxidation is a name reaction from the field of organic chemistry and was first observed in 1953 by Eric Boyland (1905–2002) and Peter Sims (1919–1983). Here react anilines with alkaline potassium to ortho -aminophenols.
Overview reaction
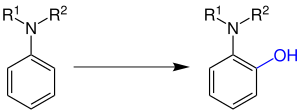
The Boyland-Sims oxidation is carried out in a basic , aqueous solution (R 1 , R 2 = alkyl group or hydrogen ):
The phenolic OH group in the ortho position shown in blue was introduced by the Boyland-Sims oxidation. If both ortho positions in the educt are occupied, a para- aminophenol is formed.
Possible mechanism
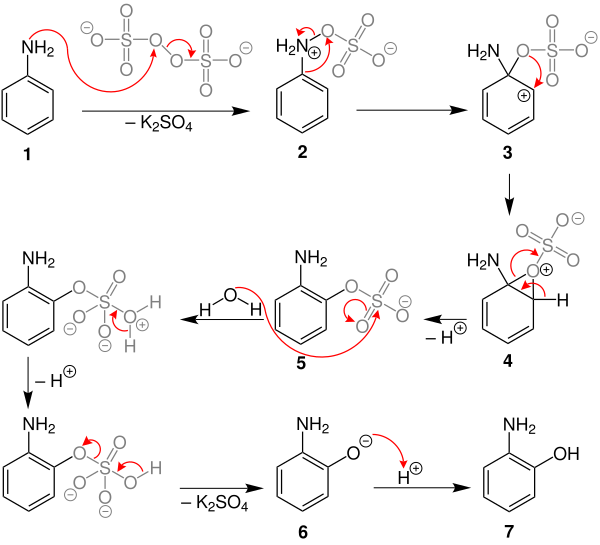
The following mechanism of the Boyland-Sims oxidation is explained in an aqueous potassium hydroxide solution by an ipso attack of the peroxide group of the potassium peroxodisulfate on aniline.
The free electron pair of the nitrogen atom of the aniline ( 1 ) attacks one of the two unstable oxygen atoms of the peroxide group of the potassium peroxodisulphate and splits off potassium sulphate . This creates a phenyl ammonium sulfate 2 . Now the ipso attack of the sulfate group takes place, through which the more stable intermediate product 3 is formed and is dearomatized . The cleavage of the double bond creates a carbenium ion in the ortho position and thus an epoxy group 4 . The hydrogen atom of epoxide group 4 splits off and rearomatizes to form ortho- aminophenyl sulfate 5 . The attack of water on the sulfate group produces the ortho- amino phenolate 6 through several internal mechanisms . The end product ortho- aminophenol ( 7 ) is formed by protonation of the phenolate .
application
The reaction is mainly used in the preparation and subsequent processing of aminophenols into azo dyes and painkillers.
literature
- Bradford P. Mundy, Michael G. Ellerd, Frang G. Favaloro Jr .: Name Reactions and Reagents in Organic Synthesis. Second Edition, John Wiley & Sons, New York 2005, ISBN 0-471-22854-0 , p. 114.
- Zerong Wang: Comprehensive Organic Name Reactions and Reagents. John Wiley & Sons, Ney Jersey 2009, ISBN 978-0-471-70450-8 , pp. 497-500.
Individual evidence
- ↑ E. Boyland, P. Sims, DC Williams: The oxidation of tryptophan and some related compounds with persulphate . In: Biochemical Journal . tape 62 , no. 4 , April 1956, p. 546-550 , PMID 13315210 , PMC 1215958 (free full text).
- ↑ Bradford P. Mundy, Michael G. Ellerd, Frang G. Favaloro Jr .: Name Reactions and Reagents in Organic Synthesis. Second Edition, John Wiley & Sons, New York 2005, p. 114.
- ^ Zerong Wang: Comprehensive Organic Name Reactions and Reagents. John Wiley & Sons, Ney Jersey 2009, p. 498.