Campaniform sensilla
Campaniform sensilles are a class of mechanoreceptors in insects that measure tension in the cuticle . Campaniform sensilles serve as proprioceptors, which record mechanical loads in the form of muscle resistance, similar to the Golgi tendon organs of vertebrates. Sensory feedback from campaniform sensillae is used to control posture and locomotion.
construction
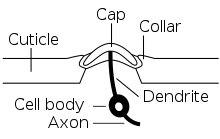
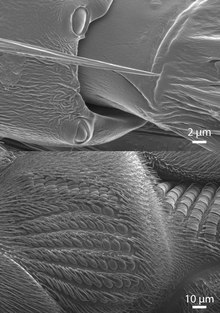
A campaniform sensillum consists of a flexible dome that is embedded in the cuticle and innervated by the dendrites of a single bipolar sensory nerve cell (see first diagram). Campaniform sensilla are often oval with long axes of about 5–10 µm (see SEM images).
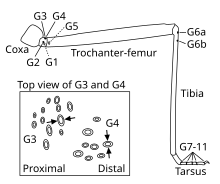
Campaniform sensilla are distributed over the body surface of many insects. Sensilles with similar orientation are often arranged in groups where high levels of tension in the cuticle are expected, including the legs, antennae and wings. For example have stick insects groups of campaniform sensilla on the trochanter , a group on the proximal femur , a group on the proximal tibia , and a small number of sensilla on the distal end of each Tarsalglieds . In two-winged birds ( Diptera ) such as the blowfly , the highest density of campaniform sensillae is found on the base of the modified rear wing, or swinging arches ( holders ), which serve to stabilize flight.
function
Campaniform sensilla activity was first recorded by John William Sutton Pringle in the late 1930s. He also recognized that the oval shape of many sensilla gives them directional selectivity. If a campaniform sensillum is compressed along its short axis as a result of cuticular deformations, the edges of the base press the cuticular dome in. This squeezes the dendrites of the sensory nerve cells and opens their mechanotransduction channels, which leads to the formation of action potentials that are passed on to the central nervous system . Campaniform sensilla report both the strength and the change in cuticular deformation.
With regard to walking control, it is assumed that sensory feedback from campaniform sensillae in the legs increases muscle activity during the stalking phase and contributes to coordination between the legs, similar to sensory feedback from Golgi tendon organs in vertebrates.
With regard to flight control, it is assumed that sensory feedback from campaniform sensillae of the mounts and wings induces compensatory reflexes that maintain balance.
Individual evidence
- ^ A b c Sasha N. Zill, Josef Schmitz, Sumaiya Chaudhry, Ansgar Büschges: Force encoding in stick insect legs delineates a reference frame for motor control . In: Journal of Neurophysiology . 108, No. 5, 2012, ISSN 0022-3077 , pp. 1453-1472. doi : 10.1152 / jn.00274.2012 . PMID 22673329 . PMC 3774582 (free full text).
- ↑ Sasha N. Zill, Sumaiya Chaudhry, Ansgar Büschges, Josef Schmitz: Directional specificity and encoding of muscle forces and loads by stick insect tibial campaniform sensilla, including receptors with round cuticular caps . In: Arthropod Structure & Development . 42, No. 6, 2013, pp. 455-467. doi : 10.1016 / j.asd.2013.10.001 . PMID 24126203 .
- ↑ J. Duysens, F. Clarac, H. Cruse: Load-Regulating Mechanisms in Gait and Posture: Comparative Aspects . In: Physiological Reviews . 80, No. 1, 2000, ISSN 0031-9333 , pp. 83-133. doi : 10.1152 / physrev.2000.80.1.83 . PMID 10617766 .
- ↑ Eiman Azim, John C. Tuthill: proprioception . In: Current Biology . 28, No. 5, 2018, ISSN 0960-9822 , pp. R194 – R203. doi : 10.1016 / j.cub.2018.01.064 . PMID 29510103 .
- ^ Sasha Zill, Josef Schmitz, Ansgar Büschges: Load sensing and control of posture and locomotion . In: Arthropod Structure & Development . 33, No. 3, 2004, pp. 273-286. doi : 10.1016 / j.asd.2004.05.005 . PMID 18089039 .
- ↑ John C. Tuthill, Rachel I. Wilson: Mechanosensation and Adaptive Motor Control in Insects . In: Current Biology . 26, No. 20, 2016, pp. R1022 – R1038. doi : 10.1016 / j.cub.2016.06.070 . PMID 27780045 . PMC 5120761 (free full text).
- ^ JWS Pringle: The Gyroscopic Mechanism of the Halteres of Diptera . In: Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences . 233, No. 602, 1948, pp. 347-384. doi : 10.1098 / rstb.1948.0007 .
- ^ John William Sutton Pringle: Proprioception in insects I. A new type of mechanical receptor from the palps of the cockroach . In: Journal of Experimental Biology . 15, No. 1, 1938, pp. 101-113.
- ^ John William Sutton Pringle: Proprioception in insects II. The action of the campaniform sensilla on the legs . In: Journal of Experimental Biology . 15, No. 1, 1938, pp. 114-131.
- ↑ SM Spinola, KM Chapman: Proprioceptive indentation of the campaniform sensilla of cockroach legs . In: Journal of Comparative Physiology? A . tape 96 , no. 3 , 1975, ISSN 0340-7594 , pp. 257–272 , doi : 10.1007 / BF00612698 ( springer.com [accessed November 5, 2019]).
- ^ KG Pearson: Central Programming and Reflex Control of Walking in the Cockroach . In: Journal of Experimental Biology . 56, No. 1, 1972, ISSN 0022-0949 , pp. 173-193.
- ↑ Sasha N. Zill, Sumaiya Chaudhry, Ansgar Büschges, Josef Schmitz: Force feedback reinforces muscle synergies in insect legs . In: Arthropod Structure & Development . 44, No. 6, 2015, pp. 541-553. doi : 10.1016 / j.asd.2015.07.001 . PMID 26193626 .
- ↑ Sasha N. Zill, Bridget R. Keller, Elizabeth R. Duke: Sensory Signals of Unloading in One Leg Follow Stance Onset in Another Leg: Transfer of Load and Emergent Coordination in Cockroach Walking . In: Journal of Neurophysiology . 101, No. 5, 2009, ISSN 0022-3077 , pp. 2297-2304. doi : 10.1152 / jn.00056.2009 . PMID 19261716 .
- ↑ Chris J. Dallmann, Thierry Hoinville, Volker Dürr, Josef Schmitz: A load-based mechanism for inter-leg coordination in insects . In: Proceedings of the Royal Society B: Biological Sciences . 284, No. 1868, 2017, S. 20171755. doi : 10.1098 / rspb.2017.1755 . PMID 29187626 . PMC 5740276 (free full text).
- ^ KG Pearson: Role of sensory feedback in the control of stance duration in walking cats . In: Brain Research Reviews (= Networks in Motion ). tape 57 , no. 1 , January 1, 2008, ISSN 0165-0173 , p. 222–227 , doi : 10.1016 / j.brainresrev.2007.06.014 ( sciencedirect.com [accessed November 5, 2019]).
- ↑ Örjan Ekeberg, Keir Pearson: Computer Simulation of Stepping in the Hind Legs of the Cat: An Examination of Mechanisms Regulating the Stance-to-Swing Transition . In: Journal of Neurophysiology . tape 94 , no. 6 , December 1, 2005, ISSN 0022-3077 , p. 4256-4268 , doi : 10.1152 / jn.00065.2005 ( physiology.org [accessed November 5, 2019]).
- ↑ Michael H. Dickinson: Haltere-mediated equilibrium reflexes of the fruit fly, Drosophila melanogaster . In: Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences . 354, No. 1385, 1999, pp. 903-916. doi : 10.1098 / rstb.1999.0442 . PMID 10382224 . PMC 1692594 (free full text).
- ↑ Amir Fayyazuddin, Michael H. Dickinson: Convergent Mechanosensory Input Structures the Firing Phase of a Steering Motor Neuron in the Blowfly, Calliphora . In: Journal of Neurophysiology . 82, No. 4, October 1, 1999, ISSN 0022-3077 , pp. 1916-1926. doi : 10.1152 / jn.1999.82.4.1916 . PMID 10515981 .