Chlorine substituted benzenes
| Chlorine substituted benzenes | |||
|
Benzene C 6 H 6 |
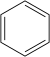
|
||
| 6 ° C | |||
| 80 ° C | |||
|
Chlorobenzene C 6 H 5 Cl |

|
||
| −45 ° C | |||
| 132 ° C | |||
|
Dichlorobenzenes C 6 H 4 Cl 2 |
 |
 |

|
| o - | m - | p - | |
| −18 ° C | −22 ° C | 53 ° C | |
| 179 ° C | 173 ° C | 174 ° C | |
|
Trichlorobenzenes C 6 H 3 Cl 3 |
 |
 |

|
| 1,2,3- | 1,2,4- | 1,3,5- | |
| 53 ° C | 17 ° C | 63 ° C | |
| 221 ° C | 213 ° C | 209 ° C | |
|
Tetrachlorobenzenes C 6 H 2 Cl 4 |
 |
 |

|
| 1,2,3,4- | 1,2,3,5- | 1,2,4,5- | |
| 47 ° C | 54.5 ° C | 139.5 ° C | |
| 246 ° C | 246 ° C | 244.5 ° C | |
|
Pentachlorobenzene C 6 HCl 5 |

|
||
| 84-87 ° C | |||
| 275-277 ° C | |||
|
Hexachlorobenzene C 6 Cl 6 |

|
||
| 231 ° C | |||
| 323-326 ° C | |||
The chlorine- substituted benzenes are derived from benzene , in which one or more hydrogen atoms are replaced by a chlorine atom . This results in 12 different compounds that differ from one another in terms of degree of substitution and symmetry. These properties play the main role here and are shown in comparison.
A distinction is made according to the number of chlorine atoms and their position on the ring:
- (Mono-) chlorobenzene C 6 H 5 Cl
- Dichlorobenzenes C 6 H 4 Cl 2 , three structural isomers (1,2-dichlorobenzene, 1,3-dichlorobenzene, 1,4-dichlorobenzene)
- Trichlorobenzenes C 6 H 3 Cl 3 , three structural isomers (1,2,3-trichlorobenzene, 1,2,4-trichlorobenzene, 1,3,5-trichlorobenzene)
- Tetrachlorobenzenes C 6 H 2 Cl 4 , three structural isomers (1,2,3,4-tetrachlorobenzene, 1,2,3,5-tetrachlorobenzene, 1,2,4,5-tetrachlorobenzene)
- Pentachlorobenzene C 6 HCl 5
- Hexachlorobenzene C 6 Cl 6
properties
Boiling points
Overall, the boiling points increase on average with each added chlorine substituent - in contrast to the methyl-substituted benzenes, which are more inconsistent - by around 30–50 ° C (80 - 132 - ø 175 - ø 211 - ø 246 - 275 - 323). The boiling points of the three isomers of the di-, tri- and tetrachlorobenzenes are close to one another and differ within a group by a maximum of 12 ° C. The symmetry is practically irrelevant here.
Melting points
With the melting points, the symmetry is particularly important. First of all, starting from benzene to chlorobenzene, the melting point drops significantly by around 50 ° C from +6 to −45 ° C - due to the introduction of a single chlorine substituent into the highly symmetrical benzene molecule.
In the di-, tri- and tetrachlorobenzenes p -dichlorobenzene ( C 2 ), 1,3,5-trichlorobenzene ( C 3 ) and 1,2,4,5-tetrachlorobenzene ( C 2 ) are the representatives with the highest symmetry.
- Dichlorobenzenes : The p -dichlorobenzene, which has the highest symmetry, has the highest melting point of 53 ° C. In contrast to the other two isomers, it is a solid.
- Trichlorobenzenes : The melting points appear rather inconsistent compared to the dichlorobenzenes. In this group, 1,3,5-trichlorobenzene is the most symmetrical representative, the melting point is 63 ° C.
- Tetrachlorobenzenes : Due to its symmetry, 1,2,4,5-tetrachlorobenzene has the highest melting point of 139.5 ° C.
- Pentachlorobenzene melts at 84-87 ° C. In comparison with the tetrachlorobenzenes, this is lower than that of 1,2,4,5-tetrachlorobenzene at 139.5 ° C due to the lower symmetry. However, it is also higher than that of 1,2,3,4-tetrachlorobenzene at 47 ° C and 1,2,3,5-tetrachlorobenzene at 54.5 ° C due to the higher degree of substitution.
- With 231 ° C, hexachlorobenzene has by far the highest melting point of the chlorine-substituted benzenes.
density
The density increases starting from the benzene (0.88 - 1.11 - ø 1.29 - ø 1.67 - ø 1.80 - 1.61 - 2.05), but shows an exception with a lower value for pentachlorobenzene .
Individual evidence
- ↑ a b c d Entry on benzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b c d Entry on chlorobenzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on o-dichlorobenzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on m-dichlorobenzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on p-dichlorobenzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on 1,2,3-trichlorobenzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on 1,2,4-trichlorobenzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on 1,3,5-trichlorobenzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on 1,2,3,4-tetrachlorobenzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on 1,2,3,5-tetrachlorobenzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b Entry on 1,2,4,5-tetrachlorobenzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b c d Entry on pentachlorobenzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
- ↑ a b c d Entry on hexachlorobenzene in the GESTIS substance database of the IFA , accessed on December 28, 2019(JavaScript required) .
literature
- Beyer / Walter : Textbook of Organic Chemistry , 19th edition, S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , pp. 445-446.