Clauson-Kaas reaction
The Clauson-Kaas reaction , named after the Danish chemist Niels Clauson-Kaas (1917-2003), is a name reaction from the field of organic chemistry and was first described in 1952 by Niels Clauson-Kaas and Zdenĕk Tyle. The Clauson-Kaas reaction enables the synthesis of N -substituted pyrroles .
Overview reaction
The Clauson-Kaas reaction is a pyrrole synthesis in which a primary amine is converted into an N -substituted pyrrole with alkoxy-substituted tetrahydrofuran (e.g. 2,5-dimethoxytetrahydrofuran) in an acidic environment .
Reaction mechanism
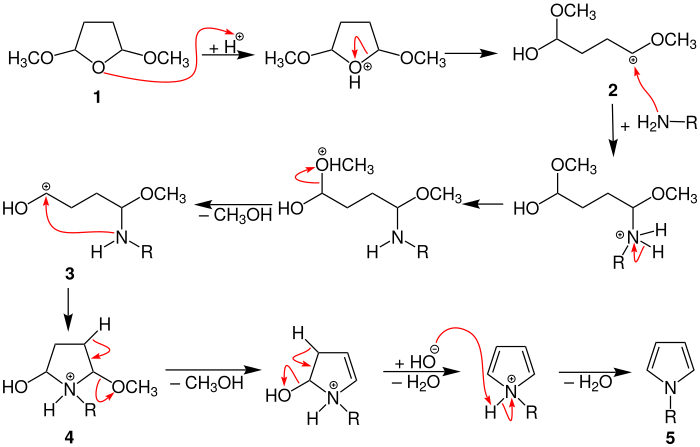
The reaction mechanism presented below is described in the literature, but has not yet been fully proven scientifically.
In the mechanism shown, methoxy radicals are used by way of example for alkoxy radicals on tetrahydrofuran.
In the first step, the 2,5-dimethoxytetrahydrofuran ( 1 ) is protonated by an acid (e.g. acetic acid ), which leads to a ring opening and the formation of carbocation 2 . In the next step, the primary amine can attack the carbocation , which leads to a proton rearrangement and then to the elimination of methanol . In the resulting carbocation 3 , the lone pair of electrons on the nitrogen atom causes a ring closure, with 4 being formed. Subsequent elimination of methanol and water and subsequent work-up with a base results in the aromatized N -substituted pyrrole 5 .
Individual evidence
- ↑ Niels Clauson-Kaas, Zdenĕk Tyle: Preparation of Cis- and Trans 2,5-dimethoxy-2- (acetamidomethyl) -2,5-dihydrofuran, of Cis- and Trans 2,5-dimethoxy-2- (acetamidomethyl) - tetrahydrofuran and of 1-phenyl-2- (acetamidomethyl) pyrroles. In: Acta Chemica Scandinavica . 6, 1952, pp. 667-670, doi: 10.3891 / acta.chem.scand.06-0667 .
- ↑ Alfred Hassner and Irishi Namboothiri: Organic Synthesis Based on Reaction name , Elsevier, 2012, ISBN 978-0-08-096630-4 , p. 30
- ^ AD Josey and EL Jenner: N-Functionally Substituted Pyrroles In: J. Org. Chem. 27, 1962, pp. 2466-2470, doi: 10.1021 / jo01054a042 .
- ^ A b Zerong Wang: Comprehensive Organic Name Reactions and Reagents , Wiley, 2009, ISBN 978-0-471-70450-8 , pp. 665-668.