Amines
When amines are organic compounds referred to, which ultimately derivatives of ammonia (NH 3 are), and in which one, two or all three hydrogen atoms of ammonia by alkyl groups or aryl groups are replaced or to more or less different heterocyclic ring systems have come together. Since amines can also contain more than one N atom, the number of possible amines is so large and their structures are so different that structures and properties cannot be presented in a single article. Cyclic amines are only mentioned in passing; therefore reference is made to the article Heterocyclen .
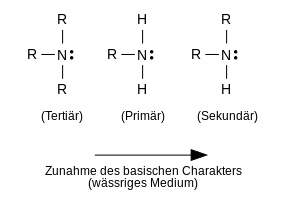
Systematics of non-cyclic amines
In general, one can distinguish between three major types of non-cyclic amines. According to the number of hydrogen atoms that have been exchanged for organic alkyl or aryl groups in ammonia, one speaks of primary, secondary and tertiary amines. Depending on the type of groups bonded to the N atom, these three types can also be referred to more precisely as aliphatic, aromatic or mixed aliphatic-aromatic amines. If the C atom bonded to the N atom is part of a CC double bond, such as e.g. B. in ethenamine , the situation is complicated, because such compounds form tautomeric forms .
Amines can also contain two N atoms (amino groups) bonded to different carbon atoms; then one speaks of diamines , which can also be of different types. If - as in the case of an aliphatic, secondary amine - there are two alkyl groups bonded to an N atom, both alkyl groups can also be closed to form a ring. Then one speaks of cyclic secondary amines, such as. B. piperidine . The ring closure can also take place with diamines, such as B. piperazine . Such cyclic amines without CC double bonds in the ring system belong to the very large and very variable group of heterocyclic amines, but behave like normal, non-cyclic amines.
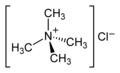
All amines can be converted into quaternary ammonium compounds by alkylation . Quaternary ammonium compounds are therefore not amines, but rather belong to another class of compounds of their own that are derived from the respective amines. The quaternary ammonium compound shown at the end of the table is therefore not an amine, but a quaternary ammonium salt of the simplest type.
| Amines and ammonium salts | |||||
|---|---|---|---|---|---|
| Types | Functional group | example | |||
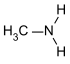
| primary amines | R-NH 2 | primary amino group | R-NH 2 | Methylamine |
|
| secondary amines | RNH-R | secondary amino group | R-NH-R | Dimethylamine |

|
| tertiary amines | NR 3 | tertiary amino group | R-NR 2 | Trimethylamine |

|
| no amines, but quaternary ammonium compounds |
NR 4 + (X - ) | quaternary ammonium group | R-NR 3 + |
Tetramethylammonium chloride |
|
| For more examples see Category: Amine and Category: Quaternary ammonium compound | |||||
properties
Chemical properties
Aliphatic amines
Like ammonia, all aliphatic amines are more or less strong bases because a proton can be attached to the lone pair of electrons on the N atom. A measure of the base strength of the amine is the base constant of the amine or the acid constant of the associated ammonium cation, expressed by the value of the base or the value of the ammonium cation. These values provide information about the position of the following equilibrium for the protonation of a primary amine by water and thus information about the base strength of the amine or the acid strength of the associated ammonium cation:
Since the ammonium cations produced by protonation are usually more soluble in water than the original amines, extraction with aqueous hydrochloric acid is the simplest method for separating the amines from other non-basic organic substances. The type of substitution and the degree of substitution can strongly influence the basicity of amines. In aliphatic amines, alkyl groups as substituents increase the electron density on the nitrogen atom because of their "electron-donating" effect ( inductive effect , + I effect ). One can therefore expect that the lone pair of electrons on the N atom will be protonated more and more easily when the number of alkyl groups on the N atom (degree of substitution) increases. However, this only applies if there are no other disturbing effects - such as B. the hydration of the amines and the ammonium cations formed by water molecules - become effective. If there are no disturbances, then one speaks of the so-called gas phase acidity . Under such anhydrous conditions, the basicity of aliphatic amines increases, as expected, with increasing substitution. This means that aliphatic amines are also stronger bases than ammonia in the following order:
Ammonia <primary <secondary <tertiary.
In the presence of water, the hydration of ammonia and the amines and ammonium cations releases heat of hydration, most of it with primary amines and least of that with tertiary amines. With increasing degree of substitution, hydration is evidently sterically hindered. These effects lead to a changed sequence of basicity in aqueous solutions:
In aqueous solution, aliphatic amines show the following order of basicity:
tertiary <primary <secondary.
The described gradations and the significantly lower basicity of tertiary amines in aqueous solutions are also reflected in the and values of ammonia and the three simplest alkylamines .
- Ammonia: 9.25; = 4.75;
- Methylamine : 10.6; = 3.4; (all primary aliphatic amines have similar values).
- Dimethylamine : 10.8; = 3.2;
- Trimethylamine : 9.8; = 4.2;
Aromatic amines
While the basicity of aliphatic amines is greater than that of ammonia because of the + I effect of the alkyl groups, the basicity of aromatic amines is significantly lower than the basicity of ammonia. Therefore, the value of the simplest aromatic amine aniline is 9.37, which is significantly higher than that of ammonia with 4.75. This shows that the –I effect of aromatic rings comes into play. Other electron withdrawing substituents on the aromatic ring system of aniline - such as. B. the nitro group in nitroanilines can drastically reduce the basicity even further.
Heterocyclic amines
The properties of cyclic amines are very different depending on the type of ring systems, which can consist of several differently linked ring systems of various types, and cannot be described generally. So behave z. B. cyclic amines with non- aromatic ring systems without double bonds, such as. B. piperidine , such as normal secondary, aliphatic amines. In contrast, the cyclic amine with a ring system and three conjugated double bonds, pyridine , does not behave like a normal tertiary aliphatic amine. Amines of this type are also bases, but have very different properties and form a new class of compounds of their own: aromatic, heterocyclic amines.
Physical and physiological properties
Aliphatic amines such as monomethylamine , dimethylamine and trimethylamine as well as ethylamine are gaseous at room temperature. Many other homologous compounds are liquid and some even higher homologous amines, such as e.g. B. Decylamine are solid at room temperature.
The simplest aromatic amine aniline is liquid. Many substituted anilines and other aromatic amines with multiple aromatic ring systems, such as. B. Naphthylamines are solid.
Due to their polarity and basicity , amines are more soluble in water than hydrocarbons with the same number of carbon atoms. The water solubility of aliphatic amines decreases with increasing length of the alkyl chains. Aromatic amines are not soluble in water. Liquid primary and secondary aliphatic and aromatic amines, are hydrogen bonds associated . Similar to alcohols , this leads to higher boiling points compared to analogous hydrocarbons.
Gaseous aliphatic amines irritate the mucous membranes of the eyes and the respiratory tract. When the skin is wetted with liquid alkylamines, chemical burns also occur. Poisoning through inhalation of higher concentrations can cause blood pressure increases and short-term cramps. Aromatic amines are not irritating because of their lower basicity and lower volatility, but are significantly more toxic than aliphatic amines, e.g. B. aniline .
Gaseous aliphatic amines have a similar to ammonia, but also have a "fishy, putrid" odor. Higher homologues and aromatic or heterocyclic amines also have odors that people perceive as unpleasant , e.g. of feces ( indole , skatole ), or of decaying meat ( cadaverine , putrescine ), of urine or old fish ( methyl , ethyl and trimethylamine ). Such compounds can arise as intermediate or end products in the anaerobic degradation of biological material, in particular proteins , or through the decarboxylation of amino acids . For the characteristic smell of sperm is spermine a linear polyamine - - with two primary and two secondary amino groups, responsible.
On the other hand, many drugs belong to the group of amines, particularly often to the subgroup of heterocyclic amines, such as. B. atropine , amphetamine , quinine , codeine and caffeine , but also drugs such. B. methamphetamine , cocaine , nicotine .
Production, creation
The idea that amines by alkylation of ammonia z. B. can easily be represented with alkyl halides is a misconception, not only because ammonia is gaseous. The alkylation of ammonia would result in mixtures of all alkylation stages, since the primary amines initially formed are even preferably further alkylated to give secondary amines, these to tertiary amines and these finally to quaternary ammonium salts.
Primary amines
The following important methods are available for the targeted preparation of primary amines:
- The Gabriel synthesis is often used. In this reaction, phthalimide is first reacted with the corresponding alkyl halide and the resulting alkyl phthalimide is then worked up with sodium hydroxide solution by hydrolysis or, better, with hydrazine by hydrazinolysis, in order to release the primary amine.
- As an alternative, alkyl halides can be reacted with sodium azide to form alkyl azides , which are then subsequently reduced to primary amines with lithium aluminum hydride .
- With chain extension, an alkyl halide is reacted with sodium cyanide to form nitrile , which is then reduced to the primary amine.
- The Hofmann method of carboxamide degradation also produces primary amines, although the carbon chain is shortened by one carbon atom.
- Another way of synthesizing primary amines with chain shortening is the Curtius reaction , in which the amine is produced by breaking down an acyl hydrazide.
Secondary amines
- Secondary amines can be prepared from primary amines by reacting the primary amine R'-NH2 with an aldehyde R-CH = O to form the imine (R-CH = N-R '), which is then reduced to the secondary amine by hydrogenation of the double bond becomes.
Tertiary amines
The Leuckart-Wallach reaction is suitable for the production of tertiary amines .
Aromatic amines
Aromatic amines are produced by nitration and subsequent reduction (e.g. aniline , toluidine ).
Technical procedures
- Technical processes for the production of amines are reactions of ammonia with alcohols (→ see alcohol amination ). B. alkylamines such as methyl and ethylamine, which then have to be separated. Aldehydes or ketones can also be reacted with ammonia, with z. B. Isopropylamines arise. Further alkylations with chloroalkanes result in particular in fine chemicals and quaternary ammonium salts.
- The reduction of nitriles provides z. B. fatty amines
- The reaction of epoxides with amines or ammonia is carried out on an industrial scale . This creates ethanolamines , isopropanolamines, etc.
- The nitration of alkanes and subsequent reduction to the alkylamine and the hydroamination of olefins are of little industrial importance. However, they are both also operated on an industrial scale.
Biological processes
- In biological processes, amines can arise from degradation and putrefaction processes through the decarboxylation of amino acids .
The peptide bound amino acids in protein (e.g. from fish ) are broken down biochemically to amines and carbon dioxide after the death of the animals . Amines are responsible for the characteristic smell of fish, which is sometimes perceived as unpleasant. Fish is often served with a slice of lemon. Like all acids, citric acid also protonates amines with the formation of citrates (salts of citric acid) and can thus reduce the amine odor.
Occurrence
- Amines occur naturally in plants, animals and humans and are then referred to as biogenic amines, which can be formed from amino acids through decarboxylation. Biogenic amines are of great importance as tissue hormones , such as B. histamine and serotonin , which arise from the amino acids histidine and tryptophan . These two amino acids belong to the basic amino acids which are characterized by additional amino groups in addition to the obligatory α-amino group. In histidine the ring of the heterocyclic amine imidazole is the additional basic group, in tryptophan the heterocyclic amine indole is the additional basic group.
- The catecholamines , which include the primary aliphatic amines dopamine and norepinephrine , can be called neurotransmitter substances . Both compounds each also contain an additional aromatic ring system. Adrenaline is very similar to noradrenaline, but is also methylated on the nitrogen atom and is therefore a secondary aliphatic amine.
- Furthermore, heterocyclic amines such as purine and pyrimidine are found as so-called nucleic bases in the basic structural components of the nucleosides in DNA.
- Amino acids, the building blocks of peptides , are neither amines nor carboxylic acids. Although they contain the functional groups of the two classes of compounds, they do not show their typical properties.
Chemical reaction
Amines react with nitrous acid to form different products depending on the type of amine used.
- Primary aliphatic and aromatic amines react to form diazonium salts , which can be used in many ways as starting materials for further syntheses.
- Secondary amines react with so-called nitrosating agents ( nitrous acid , nitrogen oxides , nitrites ), especially in an acidic environment, such as that found in the human stomach, to form nitrosamines . Since secondary amines occur in food and nitrosamines have carcinogenic effects, this reaction deserves attention.
Also tertiary aliphatic amines can react slowly to nitrosamines with elimination of an alkyl group; aromatic tertiary amines react with the electrophilic nitrosating agents on the aromatic ring system, and aromatic nitroso compounds are formed .
- As electron-rich compounds, aromatic amines are reactive reactants for electrophilic aromatic substitutions .
- Amines are reactants in numerous nucleophilic substitution reactions, for example in the formation of carboxamides , carboximides or lactams and imines . This is why amines can be used as protective groups for carbonyl compounds or as chiral auxiliaries .
- In eliminations , voluminous secondary or tertiary amines are used as proton acceptors, which are basic but not nucleophilic.
- Amines, mostly diamines or triamines, are also used as ligands in complex chemistry .
use
- Aromatic amines are used to make azo dyes .
- Amines are building blocks for agrochemicals and pharmaceuticals, as well as for surfactants , coatings and lubricants.
- In the field of foundry technology, amines are used as catalysts to accelerate the hardening process of the binders in the molding sand during core production using the cold box process.
- Amines and diamines also serve as catalysts for the production and crosslinking of polyurethanes . Their buffering effect is used when they are used as corrosion inhibitors in aqueous systems.
- Another important field of application for amines is gas scrubbing in refineries and power plants.
Detection of amines
- To detect nitrogen in an organic compound, a sodium digestion of the substance to be examined can be carried out. In the neutralized digestion solution, nitrogen can be detected as cyanide with the Lassaigne sample as Berlin blue , or, if the substance also contained sulfur, as thiocyanate with iron (III) chloride . However, this evidence is not specific to amines, but only indicates that the analyte contained nitrogen.
- Amines can often be recognized by their characteristic pungent or unpleasant odor (ammonia-like to fishy). But that is not enough for proof.
- The Hinsberg separation is carried out in order to determine the degree of substitution of the amine, i.e. whether a primary, secondary or tertiary amine is present . The amine is converted to p -toluenesulfonic acid amide :
Tertiary amines and quaternary ammonium salts do not form sulfonamides,
secondary amines form sulfonamides that are not soluble in alkali,
primary amines form sulfonamides that are soluble in alkali.
- The unambiguous identification of an unknown amine is carried out either by mass spectrometry or by means of a suitable derivative , the characteristic melting point of which is determined:
- Primary and secondary amines: The sulfonamide already obtained from the Hinsberg separation is suitable as a derivative (see above).
- Tertiary amines: Here, the precipitation of picrates is recommended.
Primary, secondary and tertiary amines can be separated chromatographically without derivatization using HPLC . The detection and quantification takes place with a mass selective detector (HPLC / MS). For the clear determination of amines with the same molar mass (e.g. diethylamine and butylamine ), the use of standard substances for calibration is recommended.
See also
literature
- Hans Beyer , Wolfgang Walter: Textbook of organic chemistry. 22nd edition. 1991, ISBN 3-7776-0485-2 .
- Author community: Organikum. 22nd edition. Wiley-VCH, Weinheim, 2004, ISBN 3-527-31148-3 .
- Karl Schwister: Pocket Book of Chemistry. Hanser Fachbuchverlag, 2004.
Remarks
Web links
- Vegetable amines .
- Explanation of the basic character of amines at TutorVista.com (English).
- Tables with pK b values of various amines.
Individual evidence
- ↑ Jonathan Clayden, Stuart Warren, Nick Greeves, Peter Wothers: Organic Chemistry . Oxford University Press, New York 2001, ISBN 0-19-850346-6 , pp. 199-200 .
- ↑ Louis Frederick Fieser , Mary Fieser: Textbook of organic chemistry. 3rd edition, Verlag Chemie, 1957, p. 254.
- ↑ Entry on nitrosamines. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.