Hinsberg separation
| Sulfonic acid amides |
| Sulphonic acid amide of a primary amine, R 1 is an aryl radical (phenyl or 4-methylphenyl) in the Hinsberg separation and R 2 is an organyl radical (alkyl radical, aryl radical, arylalkyl radical, etc.). |
| Sulphonic acid amide of a secondary amine, R 1 is an aryl radical (phenyl or 4-methylphenyl) in the Hinsberg separation, R 2 and R 3 are identical or different organyl radicals (alkyl radicals, aryl radicals, arylalkyl- Leftovers etc.). |
The Hinsberg separation , also known as the Hinsberg reaction , is named after the German chemist Oscar Hinsberg . Separation is a method in organic chemistry to separate primary , secondary and tertiary amines or in qualitative organic analysis to identify them.
application
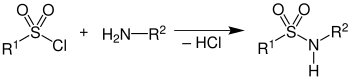
The reaction of an amine mixture with benzenesulfonic acid chloride (or - more rarely - p -toluenesulfonic acid chloride ) in the presence of sodium hydroxide leads to the formation of the corresponding sulfonic acid amides (sulfonamides). The amines react as follows:
- Primary amines form sulfonic acid amides of the formula R-SO 2 -N H R, in which the hydrogen bonded to the nitrogen is acidic . These sulfonic acid amides are acids and, after reacting with caustic soda, form sodium salts that are soluble in water .
- Secondary amines form sulfonic acid amides of the formula R-SO 2 -NR 2 that are very difficult to cleave . These products do not contain an acidic hydrogen atom, so they cannot be deprotonated by sodium hydroxide solution and are therefore insoluble in water.
- Tertiary amines do not react with the sulfonic acid chloride.
The different behavior of the amines thus enables their separation or differentiation.
Reaction mechanism
Zerong Wang describes a possible reaction mechanism on which the process is based in the book Comprehensive Organic Name Reactions and Reagents .
In the present example, benzenesulfonic acid chloride and a primary amine are used. In the first reaction step, the amine attacks the sulfur atom of the benzene sulfonic acid chloride. Intermediate product 1 is formed . From this a chloride ion splits off, which attacks anion 2 in the next step . A benzenesulfonic acid amide is formed with the elimination of hydrogen chloride.
Other uses
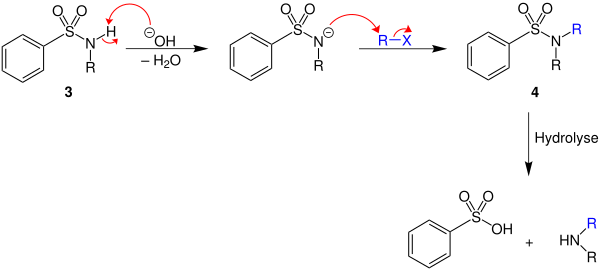
In addition to the analytical differentiation between primary, secondary and tertiary amines, the process is also suitable for the targeted synthesis of secondary amines from primary amines:
The benzenesulfonic acid amide 3 obtained from the reaction of benzenesulfonic acid chloride and a primary amine can be deprotonated by adding a base with elimination of water. If a haloalkane (R – X) is added, the benzenesulfonic acid amide 4 is formed through nucleophilic substitution . From this, benzenesulfonic acid and the desired secondary amine can be prepared by hydrolysis.
disadvantage
The process described for the synthesis of secondary amines from primary amines is a purely laboratory process. Because of the generation of stoichiometric amounts of waste products (including benzenesulfonic acid), this process has poor atom economy .
Individual evidence
- ↑ a b O. Hinsberg: About the formation of acid esters and acid amides in the presence of water and alkali. In: Reports of the German Chemical Society. 23, No. 2, 1890, pp. 2962-2965, doi : 10.1002 / cber.189002302215 .
- ↑ O. Hinsberg, J. Kessler: About the separation of the primary and secondary amine bases. In: Reports of the German Chemical Society. 38, No. 1, 1905, pp. 906-911, doi : 10.1002 / cber.190503801161 .
- ^ Hans Beyer , Wolfgang Walter : Organic chemistry. S. Hirzel Verlag, Stuttgart 1984, ISBN 3-7776-0406-2 , p. 161.
- ^ Siegfried Hauptmann : Organic chemistry. 2nd edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 493.
- ↑ a b c Zerong Wang: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 1419-1421 .