Benzenesulfonyl chloride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Benzenesulfonyl chloride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 5 ClO 2 S | ||||||||||||||||||
| Brief description |
colorless to greenish liquid with an unpleasant pungent odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 176.62 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.38 g cm −3 |
||||||||||||||||||
| Melting point |
15 ° C |
||||||||||||||||||
| boiling point |
251 ° C (decomposition) |
||||||||||||||||||
| Vapor pressure |
6.4 mbar (20 ° C) |
||||||||||||||||||
| solubility |
Decomposes in water |
||||||||||||||||||
| Refractive index |
1.553 (20 ° C, 589 nm) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Benzene sulfonyl chloride is a chemical compound from the group of organosulfur compounds .
Extraction and presentation
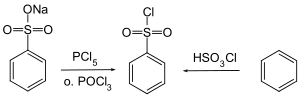
Benzenesulfonyl chloride can be obtained by reacting sodium benzenesulfonate with phosphorus pentachloride or phosphorus oxychloride and by reacting benzene with chlorosulfonic acid.
properties
Benzenesulfonyl chloride is a flammable, not very volatile, colorless to greenish liquid with an unpleasant pungent odor, which is practically insoluble in water. It decomposes when heated above 251 ° C. It reacts with water, ammonia , ethanol or phenol to form the corresponding benzenesulfonic acid derivative . With benzene , it forms diphenyl sulfone through a Friedel-Crafts reaction .
use
Benzenesulfonyl chloride is used for the Hinsberg separation of primary from secondary amines, these being converted into benzenesulfonic acid amides by reaction with the compound, the different solubilities of which are then used. It is used as an intermediate in the manufacture of benzenesulfonamides, thiophenols, glybazole and benzonitriles.
Individual evidence
- ↑ a b c d e f g h i j Entry on benzene sulfonyl chloride in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Data sheet benzenesulfonyl chloride (PDF) from Merck , accessed on December 31, 2012.
- ↑ Roger Adams and CS Marvel: Benzolsulfonyl Chloride In: Organic Syntheses . 1, 1921, p. 21, doi : 10.15227 / orgsyn.001.0021 ; Coll. Vol. 1, 1941, p. 84 ( PDF ).
- ^ Raj K. Bansal: A Textbook Of Organic Chemistry . New Age International, 2003, ISBN 81-224-1459-1 , pp. 632 ( limited preview in Google Book search).
- ↑ Richard Göttlich, Siegfried Schindler: Basic chemical internship in the minor . Pearson Deutschland GmbH, 2011, ISBN 3-86894-030-8 , p. 225 ( limited preview in Google Book Search).
- ↑ Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens, 5th Edition . 2000, ISBN 0-8155-1553-7 , pp. 334 ( limited preview in Google Book search).