Nitroanilines
| Nitroanilines | |||||
| Surname | 2-nitroaniline | 3-nitroaniline | 4-nitroaniline | ||
| other names |
o -nitroaniline, 1-amino-2-nitrobenzene |
m -nitroaniline, 1-amino-3-nitrobenzene, CI 37030 |
p -nitroaniline, 1-amino-4-nitrobenzene |
||
| Structural formula |

|

|
|
||
| CAS number | 88-74-4 | 99-09-2 | 100-01-6 | ||
| PubChem | 6946 | 7423 | 7475 | ||
| Molecular formula | C 6 H 6 N 2 O 2 | ||||
| Molar mass | 138.13 g mol −1 | ||||
| Physical state | firmly | ||||
| Brief description | yellow or orange, almost odorless, crystalline powder | ||||
| Melting point | 71 ° C | 114 ° C | 147 ° C | ||
| boiling point | 284 ° C | Decomp. | 336 ° C | ||
| Vapor pressure | 0.26 Pa (30 ° C) | 0.15 Pa (30 ° C) | 0.02 Pa (30 ° C) | ||
| 1.89 Pa (50 ° C) | 0.31 Pa (50 ° C) | 0.05 Pa (50 ° C) | |||
|
p K S value of the conjugate acid BH + |
−0.26 | 2,466 | 1.00 | ||
| solubility | 1.1 g / l (20 ° C) | 1.2 g / l (24 ° C) | 0.5 g / l (20 ° C) | ||
| Slightly soluble in water, soluble in ethanol, ether and chloroform | |||||
|
GHS labeling |
|
||||
| H and P phrases | 301 + 311 + 331-373-412 | ||||
| no EUH phrases | |||||
| 261-273-280-301 + 310-311 |
273-280-304 + 340 302 + 352-309 + 310 |
||||
The nitroanilines (rarely: nitroanilines , aminonitrobenzenes ) are aromatic compounds that are derived from both aniline and nitrobenzene . The different arrangement ( ortho , meta or para ) of the substituents results in three constitutional isomers with the empirical formula C 6 H 6 N 2 O 2 .
presentation
3-nitroaniline can be obtained by:
- Reduction of 1,3-dinitrobenzene with sodium sulfide in aqueous solution
2-Nitroaniline can be obtained together with 4-Nitroaniline by:
- Nitration and hydrolysis of acetanilide (a protective group is required, otherwise oxidation also occurs in addition to nitration)
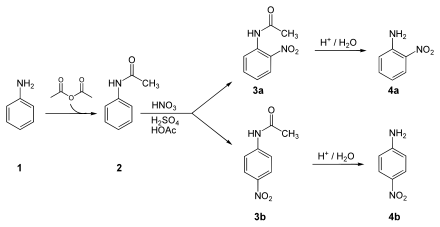 Synthesis of 2-nitroaniline or 4-nitroaniline:
Synthesis of 2-nitroaniline or 4-nitroaniline:
The NH 2 group of the aniline ( 1 ) is protected by means of acetic anhydride , resulting in acetanilide ( 2 ). This is nitrated under the action of sulfuric acid , nitric acid and acetic acid to form 2-nitroacetanilide ( 3a ) or 4-nitroacetanilide ( 3b ). Finally, the acid-catalyzed cleavage of the protective group takes place, so that 2-nitroaniline ( 4a ) or 4-nitroaniline ( 4b ) is obtained.
- Heating of 2- or 4- chloronitrobenzene with alcoholic ammonia
- Sulphonation , nitration and hydrolysis of oxanilide and acetanilide
Worldwide production is around 20,000 to 25,000 tons per year.
properties
The nitroanilines form yellow or orange crystalline powders. They are sparingly soluble in water, soluble in ethanol, ether and chloroform.
Basicity
Compared to aniline, the nitroanilines are significantly less basic, which is expressed in the lower p K S values of the conjugated acids BH + indicated in the table . The reason for this is the electron-withdrawing effect of the nitro group ( −M and −I effect ), which reduces the electron density on the nitrogen of the amino group and thus makes it difficult to take up a proton.
Melting points
The melting points show clear differences. The 2-nitroaniline having the lowest melting point, as there is an intra -molecular hydrogen bond can be formed. In contrast, the other two isomers form intermolecular hydrogen bonds. Due to its symmetry, 4-nitroaniline has the highest melting point.
use
The nitroanilines are used almost exclusively as intermediate products in the production of other organic compounds. So z. B. the phenylenediamines by hydrogenation of the nitro group and nitrobenzonitriles by diazotization .
4-nitroaniline is e.g. B. Starting point for the synthesis of the azo dye pararot :
 Synthesis of para-red starting from 4-nitroaniline ( 1 ). After the action of sulfuric acid and sodium nitrite, this reacts to form a diazonium salt ( 2 ), which is coupled with 2-naphthol to form parrot ( 3 ).
Synthesis of para-red starting from 4-nitroaniline ( 1 ). After the action of sulfuric acid and sodium nitrite, this reacts to form a diazonium salt ( 2 ), which is coupled with 2-naphthol to form parrot ( 3 ).
4- Nitroaniline was used as a fast red GG base and 2-nitroaniline as a real orange GR base with naphthol AS for developing dyes , and the corresponding stabilized diazonium salts were marketed as real red GG salt or real orange GR salt .
Derivatives
Some derivatives of 3-nitroaniline taste very sweet. The 1-propoxy-2-amino-4-nitrobenzene has a sweetening potency of 4100 in terms of sucrose and was for a time under the name Ultrasüß P-4000 as a sweetener used, but from toxicological reasons irrelevant.
safety instructions
Nitroanilines are poisonous. If nitroanilines come into contact with organic substances and are exposed to moisture, spontaneous ignition can occur.
See also
Individual evidence
- ↑ a b c d Entry for CAS no. 88-74-4 in the GESTIS substance database of the IFA , accessed on October 24, 2018(JavaScript required) .
- ↑ a b c d Entry for CAS no. 99-09-2 in the GESTIS substance database of the IFA , accessed on October 24, 2018(JavaScript required) .
- ↑ a b c d Entry for CAS no. 100-01-6 in the GESTIS substance database of the IFA , accessed on October 24, 2018(JavaScript required) .
- ↑ H: Félix-Rivera, ML Ramírez-Cedeño, RA Sánchez-Cuprill, SP Hernández-Rivera: Triacetone triperoxide thermogravimetric study of vapor pressure and enthalpy of sublimation in 303-338 K temperature range , in: Thermochim. Acta , 2011 , 514 , pp. 37-43 ( doi: 10.1016 / j.tca.2010.11.034 ).
- ↑ CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ^ Beyer / Walter: Textbook of Organic Chemistry , 19th edition, S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , pp. 533, 535-536.
- ↑ Louis Ehrenfeld, Milton Puterbaugh: o- Nitroaniline In: Organic Syntheses . 9, 1929, p. 64, doi : 10.15227 / orgsyn.009.0064 ; Coll. Vol. 1, 1941, p. 388 ( PDF ).
- ↑ JR Mohrig, TC Morrill, CN Hammond, DC Necker: Synthesis 5: Synthesis of the dye Para Red from aniline ; in: Experimental Organic Chemistry Freeman: New York, NY, 1997; Pp. 456-467.
- ↑ M. Satake, Y. Mido: Chemistry of Color . Discovery Publishing House, New Delhi 1995, ISBN 81-7141-276-9 , pp. 69 ( limited preview in Google Book search).
- ↑ Data sheet 4-Nitrobenzenediazonium tetrafluoroborate from AlfaAesar, accessed on January 29, 2019 ( PDF )(JavaScript required) .
- ↑ Fast Orange GR Salt data sheet from Sigma-Aldrich , accessed on January 29, 2019 ( PDF ).
- ↑ H.-D. Belitz et al .: Textbook of Food Chemistry , 5th ed., Springer, Berlin et al. , 2001. P. 432.



