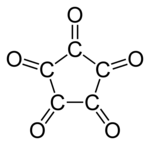
Cyclopentane pentone
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Cyclopentane pentone | |||||||||
| other names |
Leuconic acid |
|||||||||
| Molecular formula | C 5 O 5 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 140.05 g mol −1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Cyclopentane pentone is an unstable chemical compound belonging to the group of oxo carbons . So far it has only been synthesized in traces as a pure substance.
The compound exists only as a colorless tetrahydrate, whereby the water cannot be split off without the compound decomposing.
presentation
Leuconic acid is available from triquinoyl , the hydrate of hexaketocyclohexane ; with a base there is a benzilic acid rearrangement . This is followed by acidification and heating and oxidizing the croconic, the enediol of Tetraketohydroxycyclopentan .
The tetrahydrate of the compound was first synthesized by Heinrich Will in 1861 .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Detlef Schröder, Helmut Schwarz, Suresh Dua, Stephen J. Blanksby, John H. Bowie: Mass spectrometric studies of the oxocarbons CnOn (n = 3–6). In: International Journal of Mass Spectrometry. 188, 1999, p. 17, doi : 10.1016 / S1387-3806 (98) 14208-2 .
- ^ A b Frank C. Whitmore: Organic Chemistry, Volume One Part I: Aliphatic Compounds Part II: Alicyclic Compounds . Courier Corporation, 2012, ISBN 978-0-486-60700-9 , pp. 549 ( limited preview in Google Book search).
- ^ WW Pigman: The Carbohydrates Volume 1A Chemistry and Biochemistry . Elsevier, 2012, ISBN 0-323-13833-0 , pp. 537 ( limited preview in Google Book search).
- ^ History of Chemistry . Macmillan International Higher Education, 1964, ISBN 1-349-00554-1 , pp. 795 ( limited preview in Google Book search).
- ^ H. Will: Contribution to the knowledge of croconic acid. In: Annals of Chemistry and Pharmacy. 118, 1861, p. 177, doi : 10.1002 / jlac.18611180204 .